At present, COVID-19, new coronavirus pneumonia caused by SARS-CoV-2, is widely spread worldwide and has caused more than 100,000 deaths. However, there are currently no specific drugs to prevent or treat. Since the outbreak, researchers around the world have been working hard to study SARS-CoV-2, looking for effective treatment/prevention methods to cope with the global healthcare crisis brought about by COVID-19.
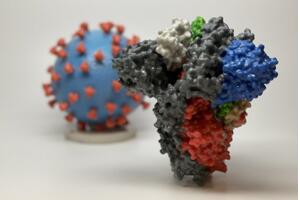 Figure 1: 3D print of a spike protein of SARS-CoV-2. The spike protein (foreground protein) allows the virus to enter and infect human cells. On the virus model, the virus surface (blue) is covered with spike protein (red) (from NIH).
Figure 1: 3D print of a spike protein of SARS-CoV-2. The spike protein (foreground protein) allows the virus to enter and infect human cells. On the virus model, the virus surface (blue) is covered with spike protein (red) (from NIH).
It is worth noting that the COVID-19 research vaccine jointly developed by NIAID and Moderna, Inc. has entered Phase 1 clinical trials in March to evaluate the safety, reactogenicity, and immunogenicity of the vaccine. The vaccine is called mRNA-1273. It is a novel mRNA-based vaccine encapsulated by nano-liposomes (LNP). It can encode the full-length, pre-fused, stable (S) protein of SARS-CoV-2 (figure1).
In addition, on April 10, Pfizer released news that it has screened a lead compound that inhibits the activity of 3CL protease of the SARS-CoV-2. At the same time, the mRNA vaccine developed by Pfizer and BioNTech is expected to start clinical trials at the end of April.
What Is an mRNA Vaccine?
The purpose of the mRNA vaccine is to produce a virus-like but harmless protein in human cells, to simulate the process of viral infection, and to stimulate the body to resist the protein and the antibody of the actual virus itself, thereby achieving a preventive effect.
mRNA Shows Unprecedented Advantages
Compared with traditional vaccines (recombinant proteins and monoclonal antibodies), the selection and testing cycle of mRNA vaccines is significantly shortened. In theory, this vaccine can be delivered to patients faster than traditional methods. At the same time, because the mRNA is easily degraded by RNase, it will not be integrated into the genome and has good biological safety. These advantages undoubtedly make the mRNA vaccine one of the most promising tools for easing the tension of the new global crown.
However, there are still many unknown issues in the research and clinical stages that require further research, such as vaccines and effective delivery and stability. The safety and effectiveness of mRNA vaccines will take longer to be better determined.
Seek Cooperation
BOC RNA, branch of BOC Sciences, focuses on RNA drug development and can provide you with comprehensive services to support your mRNA vaccine development. We are doing our best to explore various options, using our expertise and advanced technology to help provide the society with the treatment of COVID-19.
tRNA with UAAs refers to tRNA molecules enzymatically charged with non-canonical amino acids, enabling site-specific incorporation of novel residues into proteins for advanced functional and structural studies.
UAAs are delivered to target codons using engineered aminoacyl-tRNA synthetases (AARS) and orthogonal tRNA pairs, ensuring precise and efficient translation-based incorporation.
This technology enables modification of protein function, introduction of chemical handles for imaging or crosslinking, and exploration of structural properties beyond natural amino acid capabilities.
Yes, both single-site and multi-site incorporation are achievable, supporting complex protein modifications and sophisticated functional regulation in research applications.
Incorporation is confirmed using advanced analytical techniques including MALDI-MS, affinity purification, and high-throughput sequencing to ensure modification accuracy and efficiency.
We support diverse UAAs including those with azides, alkynes, ketones, aryl halides, and alkenes, enabling broad chemical, structural, and functional research applications.