The primary factor influencing mRNA stability is RNase activity. The RNases group includes enzymes that destroy RNA molecules through two mechanisms where exoribonucleases remove nucleotides from RNA ends while endoribonucleases perform internal RNA cleavage. These enzymes degrade mRNA quickly which leads to a substantial decrease in both its half-life and effectiveness. RNase A demonstrates its powerful catalytic activity by reducing RNA half-life from months down to microseconds through RNA cleavage across more than 12 orders of magnitude. RNases exhibit different specificities and activities where certain types need metal ions as cofactors like RNase H while others target double-stranded nucleic acid regions as seen with Ribonuclease III. The integrity of mRNA during production, storage, and delivery depends heavily on effective control of RNase activity. To reduce RNase activity researchers employ RNase-free reagents along with strict laboratory cleanliness and mRNA chemical modifications that improve resistance to breakdown.
The surrounding pH levels and ionic conditions play a critical role in determining mRNA stability. RNA hydrolysis speeds up at mildly alkaline pH conditions such as pH 8.0 because hydroxide ions become more available to activate the 2′-hydroxyl group which functions as a base. RNA degradation through hydrolysis becomes possible even at low pH values. Divalent metal ions such as Mg2+ stabilize RNA structures by neutralizing phosphate charges and reinforcing both secondary and tertiary formations. Specific metal ions such as Cu2+, Fe2+, and Co2+ act as catalysts in RNA degradation through oxidative stress which breaks phosphodiester bonds. RNA structures need an optimal ionic strength between 10 mM and several hundred mM to maintain structural stability and prevent degradation. Appropriate pH levels and ionic conditions must be maintained during mRNA production, storage and delivery to preserve its integrity and functionality.
Temperature significantly impacts mRNA stability. Higher temperatures speed up RNA hydrolysis through an increase in molecular energy and a decrease in the strength of weak molecular interactions. RNA molecules become more stable in low temperatures because the reduction in molecular motion strengthens their interactions. Optimal mRNA storage conditions require temperatures between -20°C and -80°C. The stability of some viral RNAs becomes compromised when temperatures fall below 0°C or exceed the range of 50–60°C. Maintaining strict temperature control throughout production and delivery processes prevents the degradation of mRNA and preserves its therapeutic effectiveness.
Physical shock together with temperature represents a crucial environmental factor that influences vaccine stability. Scientists conducted studies to determine commercial COVID-19 mRNA vaccine stability when subjected to continuous movement. The mRNA vaccines showed no significant changes when exposed to this particular environment. The experiments displayed alterations in mRNA integrity and the presence of degraded RNA fragments when shaking intensity reached 180 minutes. The study on the BNT162b2 vaccine demonstrated that vigorous shaking along with vibration and repeated syringe draw-and-release actions led to an enlargement of BNT162b2 lipid nanoparticles particle size and altered polymer dispersity index with substantial mRNA release from the lipid nanoparticles along with free mRNA loss that undermines the vaccine's protective capacity.
Maintaining the integrity of mRNA requires the use of RNase-free reagents, water, and consumables. RNases function as enzymes that quickly break down RNA molecules which naturally occur on surfaces as well as in reagents and human skin. To protect their experiments from RNase contamination, scientists utilize pipette tips and tubes that are free of RNase. A clean laboratory environment requires researchers to routinely disinfect surfaces and equipment with materials that deactivate RNase. Disposable gloves must be worn by researchers every time they handle mRNA to prevent surface contamination. Experimentation with RNA requires protection against enzymatic degradation which scientists can achieve through the application of RNase inhibitors.
Precise regulation of pH and ionic conditions is essential for maintaining mRNA stability. Basic conditions enhance mRNA vulnerability to hydrolytic breakdown thus storage conditions must remain neutral to slightly acidic. To avoid rapid RNA degradation through elevated temperature exposure during processing and handling steps it is essential to store mRNA samples on ice. Mg2+ and other divalent metal ions stimulate RNA degradation yet mRNA remains stable if chelating agents such as EDTA are applied. Store mRNA with RNase-free water or TE buffer to avoid degradation.
Temperature significantly impacts mRNA stability. The process of RNA decomposition accelerates in warmer conditions as both RNA hydrolysis activities and RNase enzyme functions become heightened. Storing mRNA samples at ultra-low temperatures like -70°C or -80°C halts enzymatic activity and reduces the likelihood of degradation which enables researchers to preserve these materials for extended periods. During short-term storage researchers store mRNA samples at -20℃ since this temperature ensures sample stability yet allows for easy access. The transportation of mRNA through areas without consistent electricity depends on dry ice because it maintains ultra-low temperature preservation. Handling dry ice properly is essential since it sublimates into CO2 gas which can cause health issues if inhaled without sufficient ventilation.
The engineering of mRNA sequences plays a vital role in enhancing mRNA stability as well as its functional performance. mRNA sequence construction significantly impacts its stability and also influences both translation efficiency and immunogenicity. The substitution of rare codons in mRNA sequences with more common synonymous codons enhances translation efficiency. The cellular machinery recognizes frequently used codons more efficiently which leads to higher protein expression levels. The stability of mRNA improves while immunogenicity decreases through the optimization of its GC content. The mRNA secondary structure plays a role in determining both its stability and translation efficiency. A stable mRNA structure increases its durability against enzymatic degradation. Efficient translation requires proper balance of structural stability because overly structured regions may prevent ribosome attachment and scanning. Sequence motifs present in mRNA molecules can activate immune responses which result in the degradation of mRNA and loss of therapeutic effectiveness. The immunogenicity of mRNA decreases when these motifs are avoided which leads to increased stability and therapeutic effectiveness.
The untranslated regions (UTRs) of mRNA are essential determinants in controlling both stability and translation efficiency. The performance of mRNA-based therapies improves significantly through UTR optimization. The 5′ untranslated region controls how ribosomes bind to mRNA and starts translation. Researchers need to prevent GC-rich secondary structures and AUG codons to maintain proper ribosome binding. The addition of ribosome binding sites like the Kozak sequence promotes improved translation efficiency. The choice of UTRs from genes with high expression levels like human α- or β-globin results in better mRNA stability and translation. The process of optimizing the 3′ UTR results in mRNA stability and efficient translation while minimizing immune responses. mRNA molecule stabilization requires embedding AU-rich elements (AREs) because these elements recruit RNA-binding proteins that help stabilize the mRNA. Excessive mRNA stabilization triggers degradation by proteins like TTP thus removing destabilizing elements such as miRNA binding sites and TLR activation sequences becomes essential.
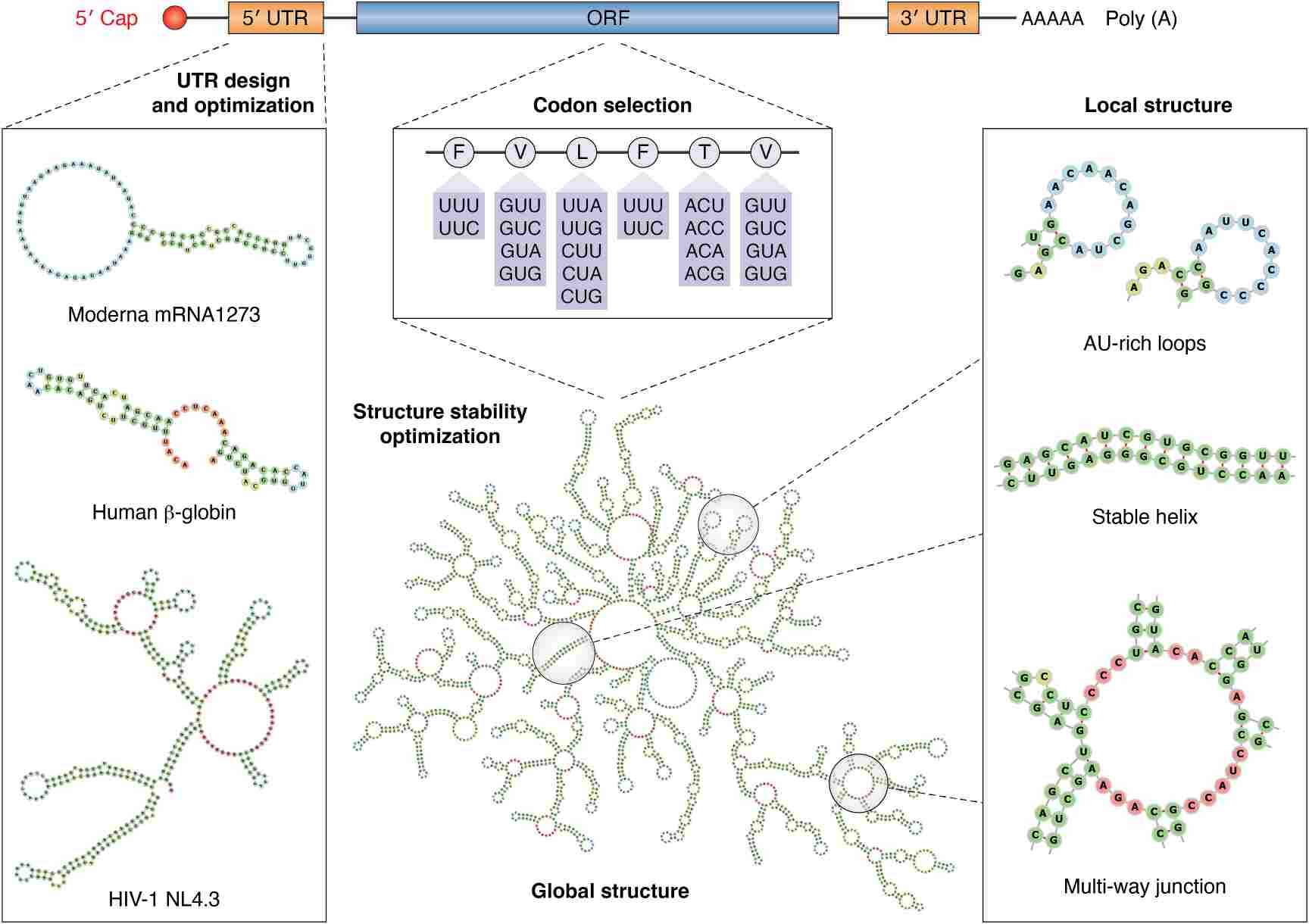 Fig.1 Illustration of in vitro transcription (IVT) mRNA components and their corresponding design/optimization processes.1
Fig.1 Illustration of in vitro transcription (IVT) mRNA components and their corresponding design/optimization processes.1
Lipid nanoparticles (LNPs) enhance mRNA stability through their formulation while also improving delivery performance. LNPs protect mRNA from enzymatic degradation and facilitate its delivery into cells: The stability of mRNA depends on choosing the correct lipid composition for LNPs. The stability of LNPs can be improved using ionizable lipids with branched saturated tail structures which lower oxidation risk. The alternative stabilizing lipid lipid-polysarcosine can replace PEG-lipids in LNPs to avoid peroxide formation that would otherwise degrade mRNA. Better manufacturing conditions for mRNA and mRNA-LNP formulations result in improved stability. The lyophilization process of freeze-drying formulations helps lower their water content which leads to reduced hydrolysis and enhanced long-term stability. Storing mRNA-LNP formulations at low temperatures such as -20℃ or -80℃ greatly enhances their stability which leads to longer shelf life. Researchers actively pursue the development of formulations that remain stable under refrigerated storage conditions of 2-8°C.
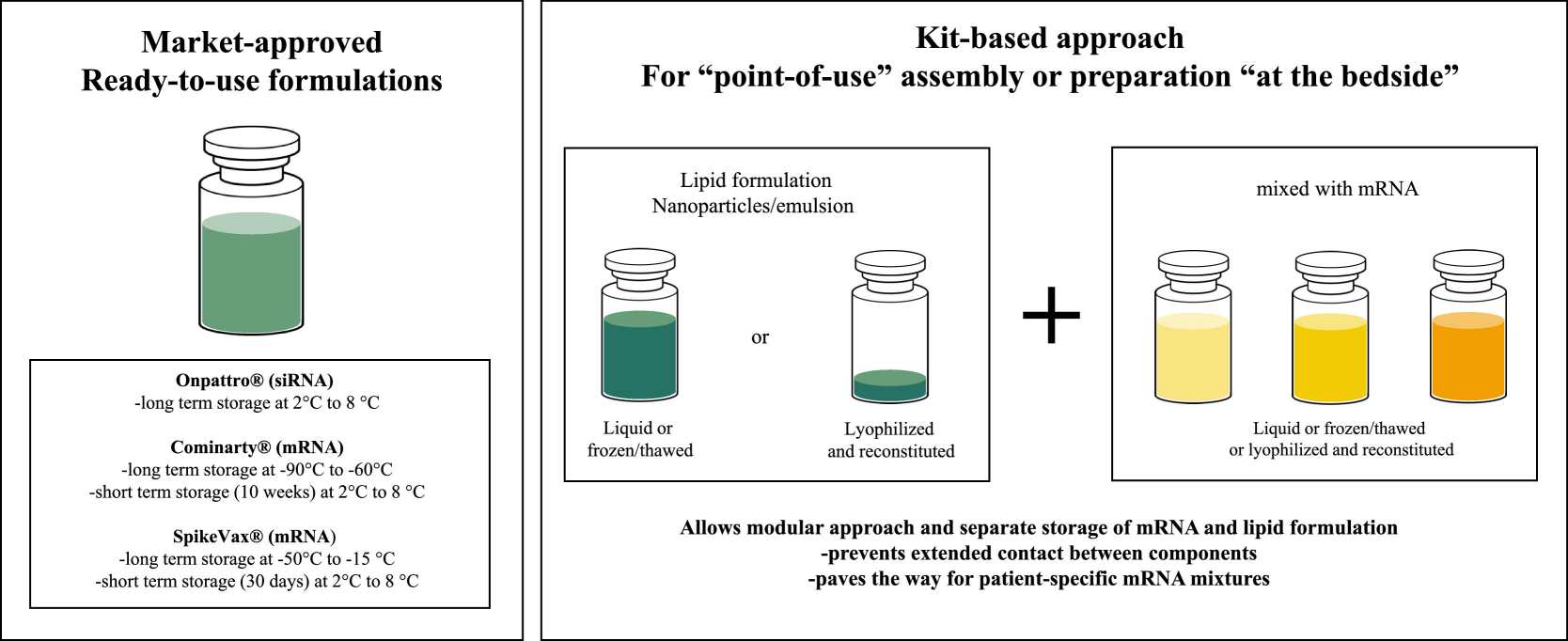 Fig. 2 Schematic of 'ready-to-use' mRNA drug product formulations currently marketed versus an approach using 'at point of use' mRNA drug product formulation by employing a 'kit-based approach'.2
Fig. 2 Schematic of 'ready-to-use' mRNA drug product formulations currently marketed versus an approach using 'at point of use' mRNA drug product formulation by employing a 'kit-based approach'.2
The primary cause of mRNA-LNP instability stems from mRNA hydrolysis. Examination of mRNA-LNP structure reveals that ionizable lipids and water together with mRNA form the core while neutral auxiliary lipids make up the outer layer of the LNP. The inclusion of water in mRNA-LNPs can trigger hydrolysis reactions. Lyophilization helps enhance the stability of mRNA-LNP formulations through its ability to minimize product water content. The mRNA product preserved through lyophilization with 10% trehalose maintained high levels of translation and protein expression after 10 months stored at 4 °C. The Moderna-developed cytomegalovirus mRNA vaccine known as mRNA-1647 is presently undergoing phase 3 clinical testing. The vaccine reportedly remains viable for a maximum of 18 months when stored at 5 degrees Celsius. Gerhardt et al. Lyophilized mRNA vaccine research results showed storage capability for over 8 months at room temperature and over 21 months when kept at 4 °C. The researchers confirmed that both the vaccine's physical properties and its protein expression levels showed no change. Ball et al. Ball et al. demonstrated that lyophilized LNP-siRNA samples remained stable for 11 months at −80 °C while retaining their gene-silencing abilities.
At BOC Sciences, we provide comprehensive solutions to help you design, test, and optimize the stability of your mRNA therapeutics. Our services support projects from early-stage research to preclinical and IND-enabling development, ensuring your mRNA maintains integrity, functionality, and potency throughout its lifecycle.
We offer a range of analytical services to assess and monitor mRNA stability under various stress conditions:
Each assay is tailored to your specific mRNA construct and intended formulation, providing the data needed for formulation refinement and regulatory compliance.
We work closely with you to develop and optimize strategies that extend the shelf life and bioactivity of your mRNA payloads:
Whether you're developing vaccines, gene therapies, or mRNA-based protein replacements, we tailor each stabilization strategy to your application and delivery route.
Beyond testing and optimization, our experts also provide strategic guidance on:
Formulation design for long-term storage or ambient-temperature applications
Cold chain compatibility for global distribution
Packaging material selection to minimize mRNA degradation during handling and transport
With deep expertise in mRNA chemistry and delivery, we ensure your therapeutic candidates are designed for durability and developed with stability in mind.
Partner with us to reduce formulation risks, accelerate development, and deliver stable, high-performance mRNA therapeutics.
Key factors include RNase susceptibility, pH and ionic conditions, temperature exposure, and mRNA sequence design including UTR optimization and structural elements.
We optimize codon usage, GC content, UTR selection, and secondary structure minimization to significantly improve mRNA stability and translational efficiency.
LNPs provide encapsulation that shields mRNA from nuclease degradation while optimized lipid compositions enhance particle integrity and stability.
We employ electrophoresis, HPLC purity analysis, LC-MS integrity verification, and functional activity assays to comprehensively evaluate mRNA stability.
Strategic incorporation of modified nucleotides enhances resistance to degradation while maintaining translational efficiency and reducing immunogenicity.
Through sequence optimization, carrier screening, and advanced formulation technologies, we develop mRNA constructs with improved stability profiles.
References