Our Diamine-scaffold based GalNAc-siRNA Conjugate Services provide a highly specialized platform for the precise design, synthesis, and validation of GalNAc-siRNA conjugates. By leveraging our expertise in chemical modification and nucleic acid delivery, we deliver customizable, high-quality, and application-ready conjugates optimized for liver-targeted RNAi therapeutics.
GalNAc-siRNA conjugates are chemically modified small interfering RNA (siRNA) molecules conjugated with N-acetylgalactosamine (GalNAc), which enhances the delivery of siRNA to hepatocytes. GalNAc is a high-affinity ligand for the asialoglycoprotein receptor (ASGPR), which is highly expressed on the surface of hepatocytes. By conjugating GalNAc to the 3' end of the siRNA sense strand, typically in a trivalent form, the resulting GalNAc-siRNA conjugates can bind specifically to ASGPR on hepatocytes and be internalized via receptor-mediated endocytosis. This allows the siRNA to enter the cell and induce RNA interference (RNAi) for therapeutic purposes.
The diamine scaffold-based GalNAc conjugate represents a novel compound designed to enhance the delivery efficiency of siRNA. Characterized by its unique structure, the diamine scaffold improves the physicochemical properties of the conjugate, allowing for a more stable and effective linkage to GalNAc. As a liver-targeted delivery system, it leverages strong interactions with the asialoglycoprotein receptor (ASGPR), enabling selective accumulation of siRNA in the liver while minimizing distribution to other organs. Compared to other conjugates, such as GalNAc-NAG37 and GalNAc-L96, the diamine scaffold conjugate demonstrates comparable or even superior siRNA delivery efficiency and gene silencing activity, particularly when connected at the 3' end. For instance, while GalNAc-NAG37 is effective in targeting ANGPTL3, the diamine conjugate exhibits enhanced inhibition of mTTR expression, demonstrating its superior potency. Overall, the incorporation of a diamine scaffold into the GalNAc conjugate structure holds significant potential for RNA-based therapies, offering new strategies in gene therapy targeting liver-associated diseases and outperforming traditional GalNAc conjugates in terms of efficiency and effectiveness.
Diamine-scaffold based GalNAc Related Products
| CAT | CAS | Name | Molecular Formula | Molecular Weight | Price |
| BRP-02497 | 2766336-04-1 | GalNAc-EDTAECA-PEG2 Phosphoramidite | C90H150N13O42P | 2117.19 | Inquiry |
| BRP-02498 | GalNAc-EDTAECA-PEG2-DMT | C106H158N12O46 | 2336.47 | Inquiry | |
| BRP-02499 | GalNAc-EDTAECA-PEG2-DMT-Suc | C110H162N12O49 | 2436.54 | Inquiry | |
| BRP-02500 | GalNAc-EDTAECA-PEG2 CPG | Inquiry | |||
| BRP-02501 | 2766335-88-8 | GalNAc-EDTAECA-PEG2-OH | C81H133N11O41 | 1916.98 | Inquiry |
Leveraging the derivatizable sites on the diamine scaffold, we can precisely modulate the steric bulk of the GalNAc moiety. This allows us to provide GalNAc-siRNA conjugate precursors tailored to meet specific requirements. Additionally, we can complete the entire GalNAc-siRNA conjugate in a single step, ensuring efficiency and precision in the synthesis process.
Customizable Diamine Backbone Length (C4-C8)
We offer customizable services for adjusting the chain length of the diamine backbone (ranging from C4 to C8). This customization enhances the binding affinity and endocytosis efficiency of GalNAc-siRNA conjugates, optimizing their performance for various applications.
Comprehensive Characterization
Our integrated characterization services include:
These comprehensive analyses ensure the quality and consistency of our GalNAc-siRNA conjugates.
One-Stop GalNAc-siRNA Validation Platform
Our one-stop validation platform for GalNAc-siRNA conjugates includes:
These tests provide critical insights into the performance and reliability of GalNAc-siRNA conjugates, supporting their development and application in therapeutic contexts.
We start with a detailed consultation to understand the client's specific needs for the GalNAc-siRNA conjugate, including desired characteristics and applications. This information guides the design and synthesis plan, ensuring the final product meets the client's requirements.
Based on the consultation, we design the GalNAc-siRNA conjugate structure, selecting the appropriate diamine scaffold and GalNAc configuration. We then develop a detailed synthesis plan, including necessary chemical modifications and purification steps to achieve the desired conjugate.
The synthesis begins with preparing the diamine scaffold and attaching GalNAc moieties. We then perform the conjugation of chemically modified siRNA to the GalNAc-diamine scaffold, ensuring high yield and purity.
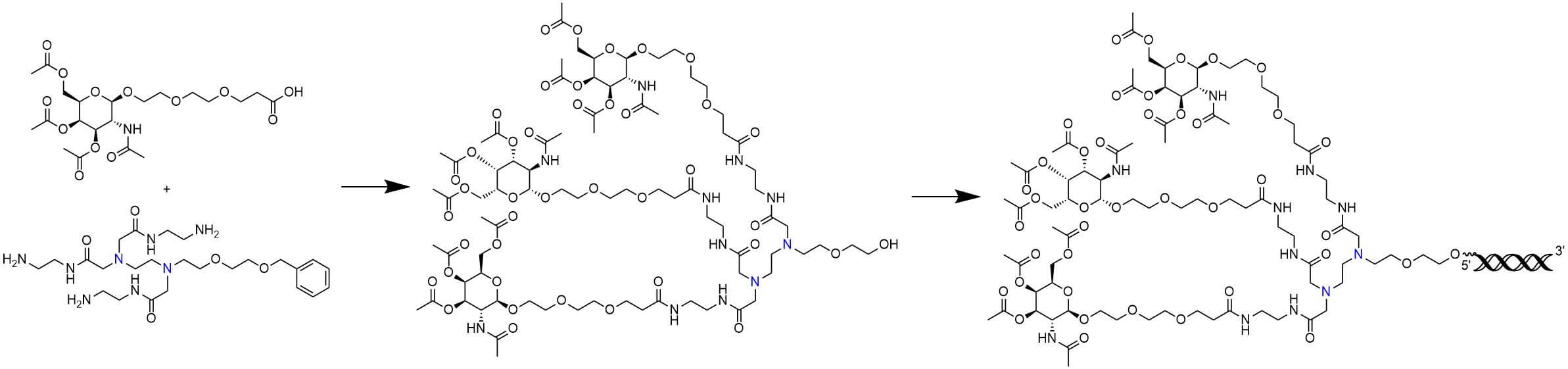 (BOC Sciences Original)
(BOC Sciences Original)
After synthesis, the conjugate is purified to remove any by-products. It is then characterized using LC-MS, HPLC, and other techniques to confirm its purity, molecular weight, and conjugation efficiency.
Clients can opt for in vitro and in vivo validation to assess the conjugate's efficacy and stability. These tests provide valuable data on the conjugate's performance in biological systems, supporting its potential application in therapeutic contexts.
We conduct final quality control checks to ensure the product meets all specified criteria. Comprehensive documentation, including analytical reports and certificates of analysis, is provided to the client, ensuring transparency and reliability.
The GalNAc-siRNA conjugate is delivered to the client with all supporting documentation. We also offer post-delivery support, including technical assistance, to help the client integrate the conjugate into their projects seamlessly.
Flexible Customization: We offer tailored synthesis to meet your specific requirements.
High-Quality Products: Our products are ensured through strict quality control, offering stability, reproducibility, and high siRNA loading efficiency.
Comprehensive and Client-Centered Service: We combine professional scientific expertise, advanced technology, premium raw materials, transparent project management, personalized customer support, and cost-effective solutions to ensure high-quality outcomes and exceptional service value.
Fast Delivery: Our streamlined logistics ensure timely delivery to meet your project deadlines.
Diamine scaffolds improve GalNAc binding, enabling more efficient liver-specific delivery for diseases such as hyperlipidemia and liver cancers.
Chemical modifications like PS linkages enhance nuclease resistance and gene silencing efficiency over conventional constructs.
Targeted delivery allows effective treatment of genetic diseases by concentrating siRNA in relevant tissues.
Strong preclinical results support advancement toward clinical trials and potential FDA-approved therapies.

The diamine scaffold provides tunable backbone lengths (C4–C8) and modifiable steric hindrance, allowing for customized conjugates with optimized binding affinity, endocytosis efficiency, and therapeutic performance.
We apply advanced characterization techniques such as LC-MS, HPLC, PS modification ratio analysis, GalNAc conjugation quantification, and endotoxin control to guarantee reproducibility and purity.
Yes. Our one-stop validation platform includes metabolic stability and pharmacodynamic (PD) durability studies, helping evaluate efficacy and long-term performance in biological systems.
Reference