High-Performance Modified mRNA for Therapeutic, Vaccine & Research Applications
Messenger RNA (mRNA) has rapidly evolved from a research reagent to one of the most versatile therapeutic modalities in modern biotechnology. Achieving optimal stability, translation efficiency, and immunogenicity control requires advanced chemical modification strategies—executed with precision, consistency, and regulatory awareness. At BOC Sciences, we provide end-to-end mRNA modification services designed for pharmaceutical companies, biotechnology innovators, CDMOs, and academic research teams. Our platform supports projects from early discovery through pre-clinical development, offering tailored chemical modifications that enhance mRNA performance across a wide spectrum of applications.
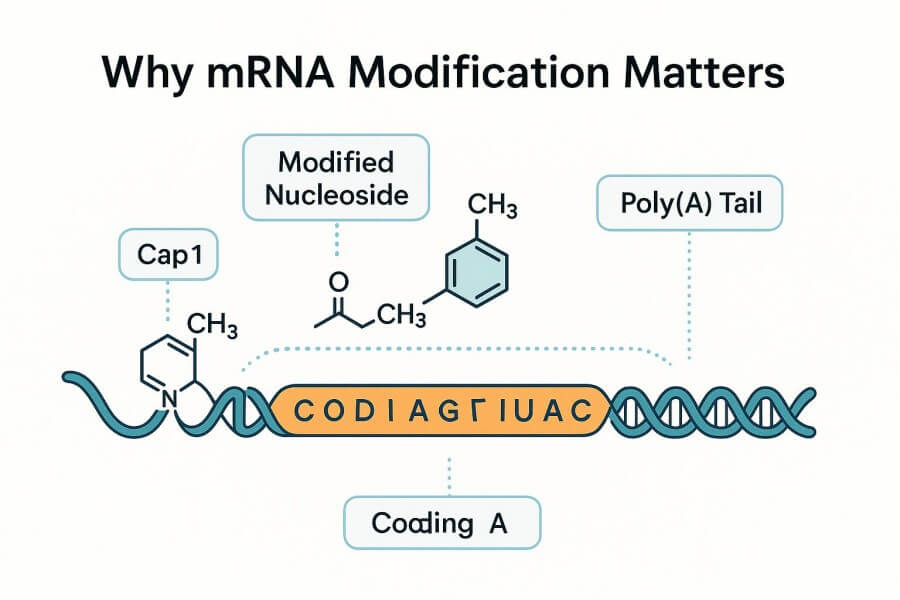 mRNA Modification Methods
mRNA Modification MethodsUnmodified mRNA can trigger innate immune responses, degrade rapidly, and produce limited protein expression. Strategic modification of nucleotides, caps, and untranslated regions (UTRs) helps solve these challenges by enabling:
Stability Enhancement: Modified nucleosides and optimized UTRs significantly improve mRNA half-life and reduce degradation.
Immunogenicity Control: Chemical modifications reduce innate immune activation while maintaining desired adaptive responses.
Translation Efficiency: Cap1 structures, optimized UTRs, and tailored Poly(A) tails boost protein expression levels.
Safety Profile: Non-integrating, transient expression minimizes genomic risk and enhances therapeutic safety.
Therapeutic Performance: Improved stability and expression profiles enable reliable in vivo efficacy for vaccines and mRNA therapeutics.
Our platform integrates the most advanced mRNA modification chemistries used in next-generation vaccines, cell therapies, gene therapies, and protein-replacement applications.
Our platform offers a complete suite of advanced mRNA modification solutions engineered to optimize expression, stability, and therapeutic performance. Each capability can be customized to match your research, preclinical, or translational development goals.
Chemical modification of nucleosides is central to regulating immunogenicity and enhancing mRNA stability.
Available Modified Nucleotides
Key Advantages
The 5' cap is essential for ribosome recognition, protection from exonucleases, and efficient translation.
Cap Options
Key Advantages
Poly(A) tail length and structure have major effects on translation and stability.
Options Available
Benefits
Untranslated regions determine mRNA's translation dynamics, cellular localization, and longevity.
Optimization Services
Benefits
We offer high-performance IVT (In Vitro Transcription) synthesis workflows designed to incorporate all selected chemical and structural modifications directly into the transcription process.
Services Provided
Benefits
Our purification and QC workflows ensure that each mRNA product meets rigorous standards for research and therapeutic development.
Services Provided
Benefits
We provide formulation and packaging options tailored to your workflow, stability requirements, and downstream application.
Services Provided
Benefits
| Capability | Options Available | Benefits | Typical Use Cases |
| Nucleoside Modification | m1Ψ, Ψ, m5C, etc. | Reduce immunogenicity | Vaccines, gene editing |
| Cap Structure | Cap0, Cap1, ARCA | Improve translation efficiency | Therapeutics |
| Poly(A) Tail | 20–300 nt, custom length | mRNA stability | Cell therapy |
| UTR Engineering | Optimized 5'UTR/3'UTR | Higher protein expression | Vaccines, research |
Assess your target application, regulatory needs, and modification strategy.
Align on technical specifications, timelines, and deliverable formats.
Enhance ORF, UTRs, poly(A) length, and codon usage based on your objectives.
Select appropriate nucleoside, cap, and structural modifications for performance.
Execute in vitro transcription using high-purity enzymes and modified nucleotides.
Integrate structural elements (Cap1, modified bases, optimized UTRs) at scale.
Purify using chromatography-based or enzymatic workflows to minimize dsRNA and impurities.
Perform full QC including purity, capping efficiency, integrity, endotoxin, and sequence validation.
Deliver the product in RNase-free solution or lyophilized format, with customized buffers if required.
Package according to your storage, stability, and downstream application needs.
Provide certificate of analysis (CoA), documentation, and technical summaries.
Offer ongoing consultation for experimental setup, optimization, and scale-up planning.
Choosing the right RNA development partner is essential for accelerating discovery, de-risking development, and ensuring consistent performance across research and therapeutic applications. Our team delivers scientific rigor, operational reliability, and deep domain expertise across every stage of mRNA design and production.
Modified mRNA has enabled a new generation of therapeutic, vaccine, and cell-engineering technologies. Through optimized nucleotide chemistry, controlled immunogenicity, and improved translation efficiency, modified mRNA offers a highly flexible platform suitable for diverse pharmaceutical and research applications. Below, we detail each major application area.
Modified mRNA provides a safe, transient, non-integrating method for producing functional proteins directly inside target tissues.
Protein replacement therapy
Correcting genetic deficiencies (e.g., metabolic enzyme disorders)
Delivering secreted therapeutic proteins without DNA-based vectors
Regenerative medicine
Driving in vivo expression of growth factors, cytokines, or reprogramming factors
Supporting tissue repair in cardiac, hepatic, and musculoskeletal diseases
Gene editing cargos
High-efficiency, short-lived expression of Cas9, Cas12a, or base editors
Reduces long-term risks associated with DNA vectors
Why Modified mRNA Matters
N1-methyl-pseudouridine reduces innate immune sensing
Cap1 structures improve protein yield
Optimized 5'/3' UTRs enhance durability and translation
Superior safety profile compared with DNA- or viral-based delivery
(Infectious Disease & Oncology)
Modified mRNA has become the backbone of next-generation vaccine design due to its rapid development speed, adaptability, and potent immune induction.
Infectious Disease Vaccines
Rapid-response vaccines for pathogens such as influenza, RSV, CMV, Zika, malaria, and emerging viral threats
High fidelity antigen expression with tunable immunogenicity
Scalable manufacturing ideal for epidemic or pandemic scenarios
Cancer Vaccines & Immunotherapy
Neoantigen-based mRNA vaccines customized to each patient's tumor mutational profile
Shared-antigen vaccines targeting established tumor-associated antigens
Combination strategies with checkpoint inhibitors or CAR-T therapies
Why Modified mRNA Matters
Modified nucleotides reduce excessive innate activation, improving antigen presentation
Controlled expression yields superior T-cell and B-cell responses
Supports both individual (personalized) and off-the-shelf vaccine development
Modified mRNA is increasingly preferred for engineering cells ex vivo because it avoids genomic integration and supports high-efficiency transfection.
Cell Therapy Engineering
CAR-T and CAR-NK cell engineering: mRNA-encoded receptors ensure temporary expression to enhance safety
Dendritic cell reprogramming: Express cytokines, co-stimulatory molecules, or tumor antigens
T-cell functional enhancement: mRNA-expressed transcription factors or immune regulators
Gene Editing Delivery
Delivery of Cas enzymes, prime editors, or base editors as mRNA
Reduced risk of off-target effects vs DNA plasmids
Suitable for ex vitro, ex vivo, and select in vivo editing workflows
Why Modified mRNA Matters
High translation efficiency for transient but potent expression
Reduced innate responses allow better cell viability post-transfection
Integration-free delivery supports regulatory acceptance
Modified mRNA enables controlled, local, and temporary expression of regenerative factors, offering a safer alternative to viral vectors.
Regenerative Applications
Cardiac repair: VEGF-A, HGF, FGF, and other angiogenic factors
Liver regeneration: hepatocyte growth factors, anti-fibrotic proteins
Wound healing: cytokines, anti-inflammatory signals, matrix regulators
Stem cell reprogramming: transient expression of factors like Oct4, Sox2, and Nanog
Why Modified mRNA Matters
Tunable protein expression without permanent gene alteration
Reduced inflammatory signaling ensures compatibility with sensitive tissues
Suitable for both direct injection and scaffold-based delivery
Modified mRNA offers a promising approach to treat diseases caused by single-gene defects.
Examples
Enzyme deficiencies (e.g., metabolic disorders)
Structural protein deficiencies (e.g., dystrophin fragments)
Signaling molecule restoration
Why Modified mRNA Matters
Rapid development cycles — no need for viral vector design
Avoids integration risks associated with DNA gene therapy
Can be combined with LNPs or targeted delivery platforms
Modified mRNA serves as a high-performance reagent for academic and industry R&D.
Applications
High-yield protein expression for biochemical studies
Reporter mRNA for imaging, tracking, or cell viability assays
Mechanistic studies in RNA stability, translation, or immunology
Drug screening systems using mRNA-encoded targets
Why Modified mRNA Matters
Predictable, repeatable expression
Reduced background innate immune activation
Compatible with automation and high-throughput screening
Advancing an mRNA program requires a partner who understands the scientific, regulatory, and operational challenges unique to RNA therapeutics. Whether you are optimizing an early-stage research construct or preparing for pre-clinical development, our team provides the technical depth and manufacturing expertise to accelerate your work with precision. When you request a quote, our scientific advisors will work closely with you to define the optimal modification strategy—including nucleotide chemistry, cap structure, UTR architecture, poly(A) design, purity specifications, and optional formulation requirements. Every proposal is tailored to your application, delivering a clear plan for technical execution, timelines, cost structure, and scale-up options. Contact us today to receive a customized proposal for your modified mRNA project.