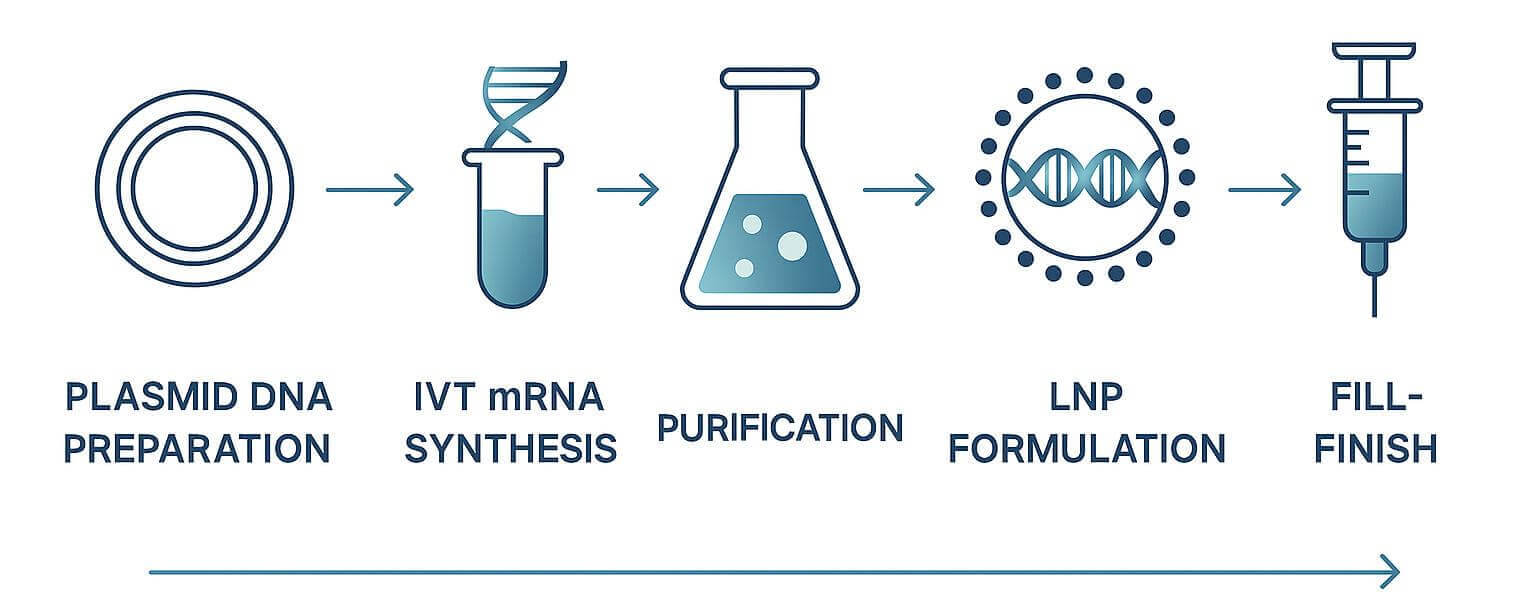
 End-to-end mRNA manufacturing workflow
End-to-end mRNA manufacturing workflow
End-to-End GMP Manufacturing for mRNA, LNP, and Drug Product Fill-Finish
Our integrated mRNA manufacturing platform provides biotech and pharmaceutical partners with a fully unified, GMP-compliant workflow encompassing sequence engineering, plasmid DNA production, high-yield IVT synthesis, advanced purification, precision LNP formulation, and aseptic drug product fill-finish. Designed for programs requiring robust scalability, high purity, and consistent performance, our end-to-end system ensures seamless coordination across every stage of production. With data-driven process control, high-throughput analytics, and tightly aligned upstream and downstream operations, we deliver reliable RNA and LNP materials that support rapid development cycles, efficient scale-up, and reproducible manufacturing outcomes—empowering partners to accelerate innovation while maintaining uncompromising quality.
High-quality plasmid DNA optimized for IVT mRNA production.
Capabilities include:
Deliverables: MCB/WCB, plasmid DNA, linearized DNA templates.
A robust, scalable in vitro transcription platform engineered for consistent high yields.
Services include:
Scale Options:
Precision microfluidic delivery systems optimized for mRNA, saRNA, and circRNA.
Key strengths:
Applications: vaccines, gene-editing, protein-replacement therapies.
Sterile manufacturing and cold-chain-ready packaging.
| Package | Includes | Best For |
| RNA Build Package | Plasmid → IVT mRNA | Rapid prototyping |
| Delivery Package | mRNA → LNP formulation | Delivery optimization |
| Full-Stack mRNA Package | Plasmid → mRNA → LNP | One-stop development |
| DP Package | LNP → Fill-Finish | Final product preparation |
Integrity and purity (CE / Bioanalyzer)
Choosing the right CDMO partner is critical for accelerating RNA program development while ensuring consistency, scalability, and uncompromising quality. Our platform combines integrated manufacturing, advanced analytics, GMP-compliant operations, and deep technical expertise to help biotech and pharmaceutical teams progress faster with confidence. We deliver a seamless end-to-end experience—from plasmid DNA and IVT mRNA to LNP formulation and final drug product—supported by data-driven process control and transparent project communication.
Advanced Infrastructure Built for Scalability, Quality, and Consistency
Our manufacturing ecosystem is engineered to support high-quality mRNA and LNP production across a wide range of research and development needs. With a combination of precision equipment, automated workflows, and fully integrated process controls, we deliver reliable, reproducible output at every scale.
This manufacturing foundation ensures your programs benefit from repeatable, scalable, and high-performance production, regardless of complexity or sequence type.
Equipment & Instrumentation
| Category | Key Equipment | Purpose |
| Upstream | Bioreactors, plasmid fermenters | pDNA preparation |
| IVT | Automated IVT reactors | High-yield transcription |
| Purification | TFF/UFDF systems, chromatography | High-purity RNA |
| LNP | Microfluidic mixers | Controlled particle formation |
| QC Labs | HPLC, CE, DLS, LC-MS | Analytical characterization |
A Platform Engineered for Innovation, Precision, and High-Performance mRNA Solutions
We are more than a supplier—our platform is built to help innovators move faster, solve complex technical challenges, and unlock new therapeutic possibilities. Our differentiators reflect deep expertise across mRNA engineering, delivery science, process design, and advanced analytics.
Unlike fragmented service providers, we offer a seamless ecosystem encompassing design, synthesis, purification, formulation, and analytics—all under one unified platform. This reduces variability, accelerates development, and enhances data continuity.
We offer more than standard LNP formulation.
Our delivery portfolio includes:
This breadth supports a wider range of research strategies and biological targets.
We specialize in challenging RNA formats, including:
Our engineering-first approach ensures efficient production and consistent quality, even for demanding constructs.
Our development workflows leverage:
This enables faster optimization cycles and superior reproducibility.
Our purification technologies ensure:
Quality is built into every stage—not just tested at the end.
Our teams integrate expertise across RNA biology, chemistry, nanotechnology, materials science, and process engineering.
This cross-functional structure empowers us to solve problems creatively and support unconventional research paths.
Our mRNA manufacturing platform supports a wide spectrum of applications across the biotechnology and pharmaceutical sectors. Each area is backed by scalable mRNA production, advanced LNP delivery, and robust quality systems.
Supporting next-generation mRNA and saRNA vaccine pipelines.
(mRNA-encoded Biologics)
Accelerating programs requiring controlled, transient expression of functional proteins.
Designed for RNA-delivered genome engineering systems.
Enabling differentiated therapeutic programs across multiple modalities.
(saRNA, circRNA, novel RNA designs)
Supporting emerging RNA modalities with specialized manufacturing and analytics.
Providing advanced formulation and nanoparticle characterization capabilities.
Helping research organizations generate material quickly for exploratory work.
Our end-to-end manufacturing platform delivers the reliability, scalability, and technical depth needed to advance modern RNA therapeutics. Whether you are optimizing early prototypes or scaling established processes, we provide the infrastructure and expertise to help you move faster—without compromising quality.