Comprehensive End-to-End mRNA Solutions
We provide fully integrated, end-to-end mRNA development, production, delivery, and analytical services to support projects from early research through IND filing and commercial scale-up. Our platform combines advanced sequence design, plasmid construction, IVT mRNA synthesis, high-purity purification, LNP formulation, and GMP manufacturing under one streamlined workflow—enabling faster timelines, superior consistency, and reduced technical risk. With deep expertise across mRNA therapeutics, mRNA vaccines, gene editing (Cas9 mRNA + gRNA), saRNA, circRNA, and mRNA-LNP delivery, we help innovators accelerate discovery and confidently advance their programs toward success. Whether you require milligram-scale research material or large-scale GMP batches, our one-stop mRNA solutions empower you to design, develop, and deliver next-generation nucleic acid medicines with speed and reliability.
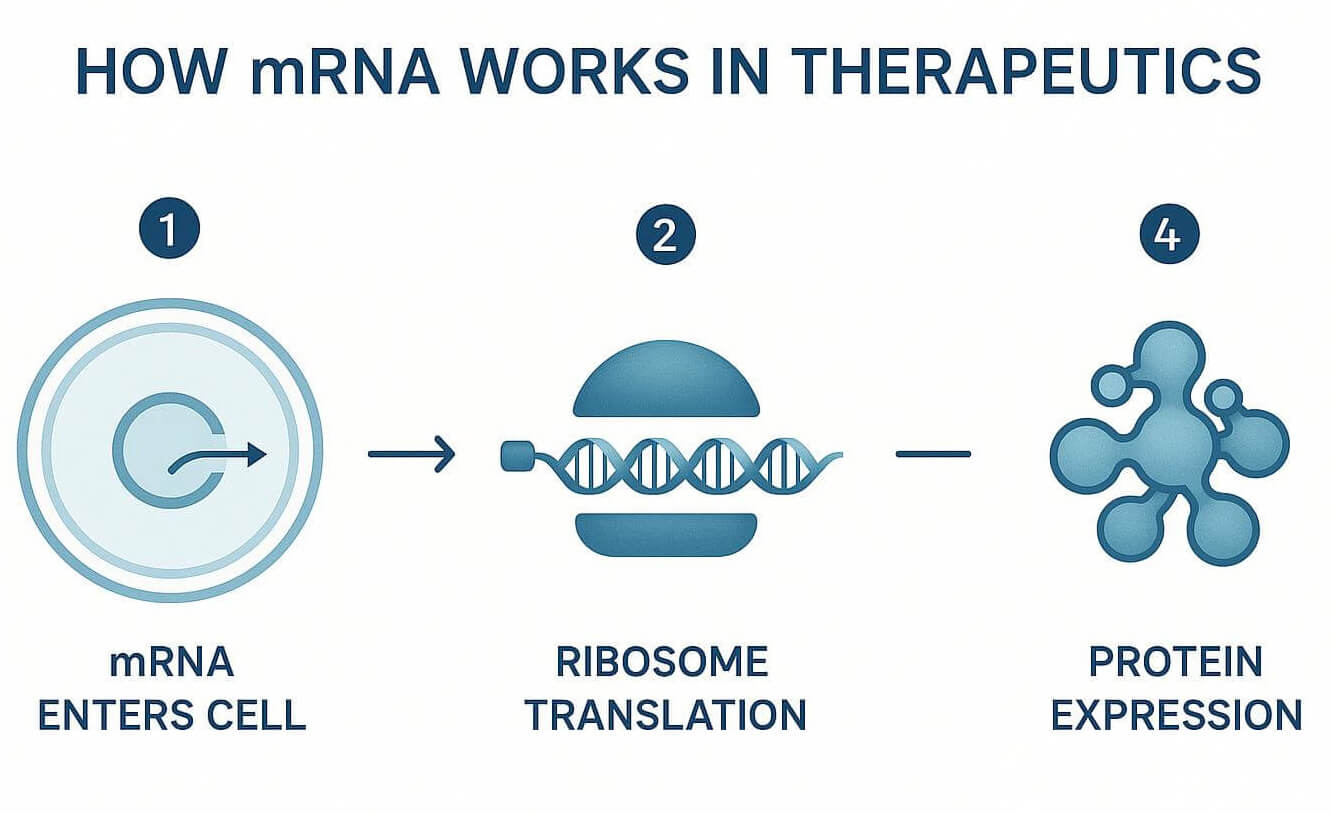 Figure 1: Schematic of an mRNA molecule
Figure 1: Schematic of an mRNA moleculeUnlocking the Next Generation of Therapeutics
Messenger RNA (mRNA) has rapidly emerged as one of the most transformative modalities in modern medicine. Its unique ability to encode virtually any protein, combined with flexible manufacturing and strong safety attributes, positions mRNA as a powerful platform for vaccines, therapeutics, and advanced cell and gene engineering.
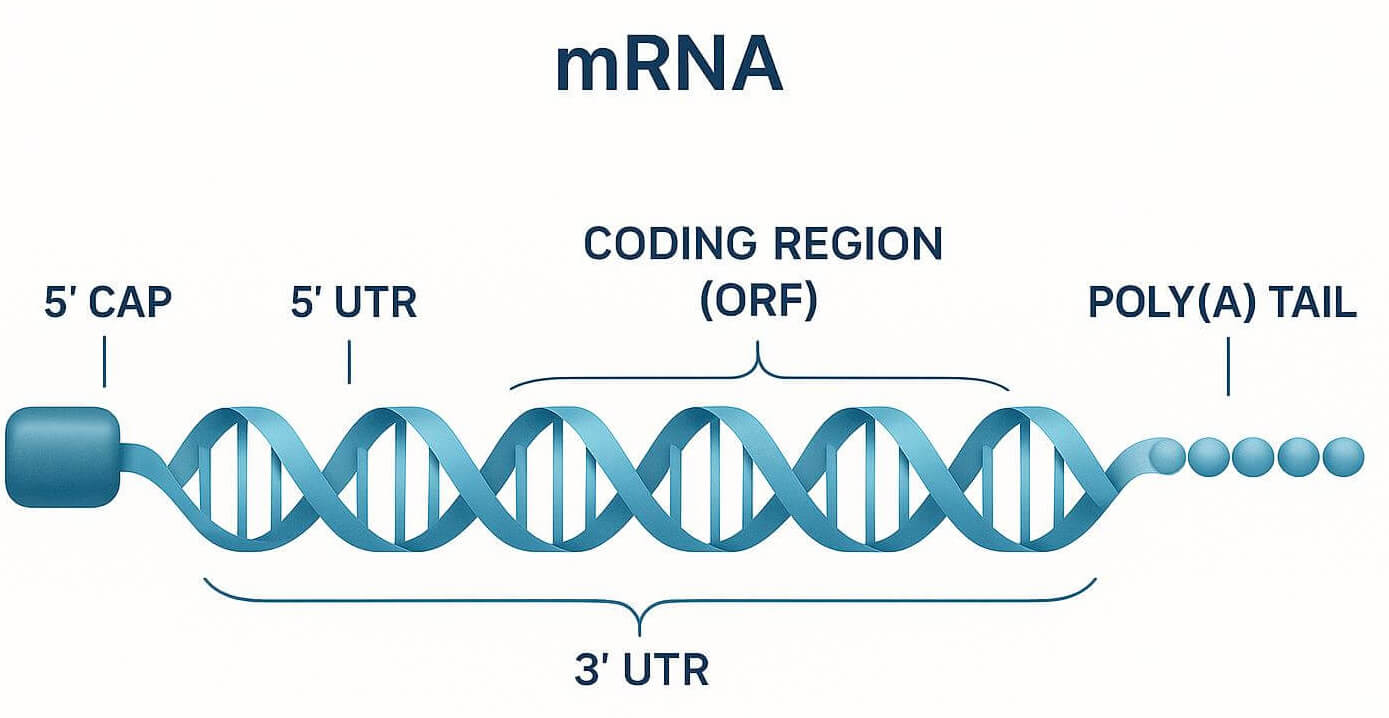 Figure 2: How mRNA works in therapeutics
Figure 2: How mRNA works in therapeutics
Comprehensive mRNA Services From Research to Commercialization
We offer a broad portfolio of customizable services to accelerate every stage of your mRNA therapeutic program.
To match your development stage and budget:
Table 1 Production Grades
| Grade | Application | QC Level | Release Documents |
| Research | Discovery & in vitro | Basic QC | Data sheet |
| Preclinical | Animal studies | Extended QC | COA |
| Pre-GMP | Tox studies | GMP-like | Batch record summary |
End-to-End mRNA Development & Manufacturing Process
Our integrated mRNA platform streamlines every phase of development—from sequence design to GMP manufacturing—ensuring high-quality, consistent, and regulatory-ready materials. Each step is supported by cutting-edge technologies, advanced analytics, and a dedicated scientific team.
We design high-performance mRNA sequences tailored to your target protein and therapeutic goals. Key capabilities include:
Our plasmid team provides rapid vector construction and template preparation for IVT. Highlights:
We synthesize high-quality mRNA using optimized IVT chemistry. Capabilities include:
To ensure therapeutic-grade purity, we apply advanced chromatographic and enzymatic purification technologies. Capabilities:
A comprehensive delivery platform designed for functional expression in vitro and in vivo. Our formulation solutions include:
Rigorous quality control ensures regulatory compliance and reliable performance. Tests include:
Full support for IND and commercial production. Features:
Advanced Engineering, Scalable Manufacturing, and Platform-Level Innovation
Our technical capabilities go far beyond standard mRNA synthesis. We operate a fully integrated mRNA engineering platform built for scalability, manufacturability, quality, and next-generation therapeutic innovation. This section highlights our core technology strengths, not individual workflow steps.
We operate an engineering-driven mRNA platform that enables rapid adaptation across diverse RNA modalities and therapeutic strategies.
Key strengths:
Our capabilities extend across multiple delivery technologies, enabling tailored solutions for each therapeutic indication.
We support:
Our analytical ecosystem provides deep structural and functional insight, surpassing basic QC requirements.
Capabilities include:
We combine molecular biology with RNA chemistry to deliver high-performance, functionally optimized mRNA constructs.
We specialize in:
Table 2 mRNA Formats & Capabilities Matrix
| RNA Type | Max Length | Modification Support | Formulation Options | Typical Use Case |
| mRNA | 20 kb | Fully supported | LNP/Polymer/Liposome | Vaccines, Protein Therapy |
| saRNA | 10 kb | Partial | LNP | Vaccines |
| circRNA | 5 kb | Custom options | LNP | Long-term expression |
| gRNA/sgRNA | 20–200 nt | N/A | Electroporation | CRISPR Editing |
Comprehensive Analytical Testing for mRNA & LNP Products
We provide a full suite of analytical assays to ensure that every mRNA product meets strict quality, safety, and regulatory standards. Our QC panel covers all critical quality attributes (CQAs) required for preclinical, GMP, and commercial use.
Table 3 QC Testing Matrix
| CQA Category | Test Method | Purpose | Included in |
| Identity | MS, sequencing | Confirm RNA structure | All grades |
| Purity | HPLC, CE | Detect impurities | Preclinical/GMP |
| Integrity | Bioanalyzer | Full-length RNA | All grades |
| dsRNA | Dot blot / ELISA | Safety & efficacy | Preclinical/GMP |
| Potency | Cell-based assay | Functional activity | Preclinical/GMP |
| LNP QC | DLS, zeta, EE% | Delivery performance | LNP batches |
Empowering Innovation Across the mRNA Therapeutic Landscape
Our end-to-end mRNA platform is purpose-built to accelerate breakthroughs across the most dynamic fields in modern medicine. From vaccines and gene editing to cell therapy and regenerative medicine, we provide the technical expertise, manufacturing capacity, and regulatory readiness needed to advance your program with confidence.
Transforming Prevention and Personalized Immunity
mRNA enables rapid, flexible vaccine development by encoding viral antigens or tumor neoantigens directly inside host cells. Whether targeting emerging infectious diseases or designing personalized cancer vaccines, mRNA offers unmatched adaptability and speed.
How we support your vaccine program:
Precision Tools for Next-Generation Genetic Medicines
mRNA is the gold standard for delivering gene editing machinery safely and transiently, minimizing off-target risks while enabling highly efficient genome modification.
Supported use cases:
How we accelerate your gene editing work:
Restoring Function Through mRNA-Encoded Proteins
mRNA provides a powerful alternative to recombinant proteins by enabling sustained, endogenous expression of therapeutic proteins without genomic integration or viral vectors.
Applicable protein classes:
Our support includes:
Redefining Biologics With In Vivo Expression
mRNA can encode complex therapeutic antibodies directly within the patient, enabling rapid response capabilities and superior pharmacokinetic profiles.
Use cases include:
Our platform capabilities:
Faster, Safer, Non-Integrating Gene Delivery
For ex vivo cell therapy, mRNA offers rapid, non-integrating expression of CARs, TCRs, co-stimulatory molecules, and editing enzymes, accelerating manufacturing without genomic disruption.
We empower your cell therapy workflows with:
Driving Tissue Repair and Functional Recovery
mRNA delivers precise, transient expression of growth factors and regenerative signals—an ideal modality for tissue repair without long-term genetic alteration.
Applications include:
Our value:
Rapid, Flexible Solutions for Ultra-Orphan Indications
For monogenic disorders, mRNA enables targeted protein restoration with minimal development timelines—an essential advantage in rare disease drug development.
Our capabilities include:
High-Precision Synthetic RNA for Analytical Innovation
Synthetic mRNA and RNA standards are essential tools for molecular diagnostics, assay development, and analytical workflows.
We provide:
Our scientific and engineering teams are committed to supporting your mRNA innovation—from sequence design and IVT production to advanced formulation, delivery optimization, and comprehensive analytical characterization. With a fully integrated platform and deep expertise across RNA modalities, we help you move faster, scale smarter, and achieve consistent high-quality results. Collaborate with a partner who understands the science and the technology behind next-generation mRNA solutions. Connect with our experts to discuss your project needs.