The development of mRNA-based therapeutics such as vaccines and gene therapies requires high-quality and reproducible mRNA products. Analytical characterization maintains its critical role in development and manufacturing processes to ensure mRNA formulations stay safe, effective, and consistent. The structure of mRNA molecules is large and single-stranded with negative charges which makes them extremely vulnerable to degradation by nucleases. The purity of mRNA determines its translational efficiency and stability along with its safety profile. The enzymatic production of mRNA vaccines/therapeutics results in the creation of incomplete mRNA molecules together with potential impurities including dsRNA. The stability of mRNA therapeutics remains vulnerable to degradation during manufacturing and storage when exposed to heat, hydrolysis, oxidation, light exposure and ribonucleases. The enzymatic IVT process enables the addition of the 5′ cap and 3′ poly(A) tail to mRNA molecules through either co-transcriptional or post-transcriptional modifications. The ribosomal binding and mRNA stability depend on the presence of the 5ʹ cap and 3′ poly(A) tail. The effectiveness of 5′ capping as well as the length and heterogeneity of the 3′ poly(A) tail work as important quality attributes (CQAs) which affect both mRNA translational efficiency and its stability during in vivo conditions. The development and performance of analytical methods to characterize mRNA DS and DP is essential for monitoring CQAs and other product attributes beyond standard stability studies.
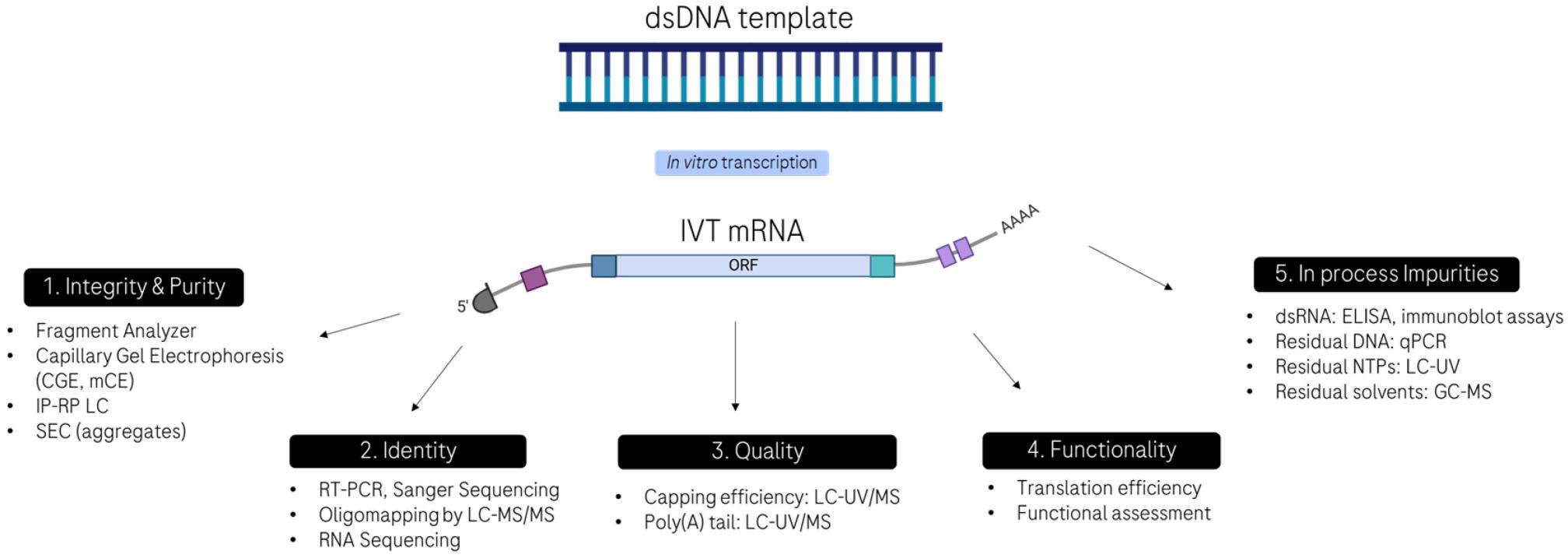 Fig. 1 Conventional analytical methods for characterizing the quality attributes of IVT mRNA.1
Fig. 1 Conventional analytical methods for characterizing the quality attributes of IVT mRNA.1
Researchers rely on UV spectroscopy as a fundamental methodology to analyze mRNA samples during nucleic acid characterization. The absorbance of nucleic acids measured across specific wavelengths provides fundamental information about both sample purity and concentration levels. The A260/280 ratio remains the primary standard researchers apply to ascertain the purity levels of nucleic acid samples. Nucleic acids demonstrate peak absorbance at 260 nm which contrasts with proteins that absorb light at 280 nm. Pure mRNA samples exhibit an A260/280 absorbance ratio of about 2.0 which indicates low levels of protein contamination. The A260/280 ratio provides a quick and dependable approach for identifying contaminants that can disrupt mRNA therapeutic applications. DNA samples show A260/280 absorbance ratios between 1.8 to 2.0 while RNA samples display ratios between 2.0 to 2.1. The presence of proteins or other contaminants becomes likely if absorbance measurements do not align with standard expected ratios.
The essential techniques of gel electrophoresis and capillary electrophoresis help scientists determine mRNA molecular size as well as structural stability. Through gel electrophoresis scientists can differentiate nucleic acids based on their size which helps them to evaluate the purity and structural condition of mRNA molecules. Capillary electrophoresis achieves superior resolution and sensitivity which establishes it as the perfect method for accurately analyzing mRNA molecule size distribution along with degradation product detection. Capillary gel electrophoresis (CGE) enables separation of impurities such as truncated and degraded products from full-length IVT mRNA transcripts. The separation method operates by moving negatively charged mRNA particles through a capillary filled with sieving gel buffer solution based on their size difference when an electric field is applied to the submillimeter-diameter capillary ranging from 20 to 70 cm in length. CGE methods have been used for quantitative analysis of mRNA size therapeutics since the 1980s for molecules up to 6500 nucleotides long which separates truncated impurities and aggregates from full-length linear IVT mRNA and more recently circular mRNA (circRNA). The right buffer compositions optimize separation efficacy and resolution which enables researchers to examine mRNA sizes across a broad spectrum. TBE and TAE buffers remain popular because their stable pH levels and ionic strength enable precise separations at 10–40 minutes intervals.
As an essential analytical tool high-performance liquid chromatography allows for versatile mRNA characterization. Recent advancements have brought substantial enhancements to LC software and hardware. Modern HPLC systems function at 1000 to 1500 bar pressures using ultrahigh-pressure liquid chromatography (UHPLC) to obtain separations that are either fast or highly resolved. Manufacturers supply a wide variety of stationary phase morphologies which encompass sub-2 μm fully porous particles, superficially porous particles along with nonporous materials. Companies are developing specialized columns optimized for RNA analysis which produce superior separation results compared to traditional LC columns. The analysis of mRNA frequently involves the use of Anion exchange (AEX) chromatography, Size exclusion chromatography (SEC), and IP-RP LC modes which prove useful for circRNAs as well. The stability of mRNA and its translation capacity depend on the efficiency of capping while translation efficiency and mRNA stability are affected by the length of the poly(A) tail. The technique of ion-pair reversed-phase HPLC (IP-RP HPLC) enables evaluation of mRNA capping efficiency through the separation of both capped and uncapped mRNA molecules. SEC analysis of poly(A) tail lengths offers valuable information about the functionality and stability of mRNA molecules.
The detection of dsRNA during mRNA characterization is essential because dsRNA can activate immune responses which diminish the effectiveness of mRNA-based treatments. Multiple detection methods for dsRNA exist including gel electrophoresis which separates dsRNA from single-stranded mRNA through size and conformation differences. Quantitative PCR and Northern blotting serve as more sensitive techniques to detect and measure dsRNA contamination. Next-generation sequencing (NGS) as an advanced technique delivers extensive data regarding the existence and distribution patterns of dsRNA within mRNA samples. The utilization of these methods is critical to guarantee mRNA products remain uncontaminated by dsRNA which reduces immunogenicity and increases therapeutic effectiveness.
MS-based methodologies have been extensively used to study mRNA structural features such as poly(A) tail length and distribution through oligonucleotide mapping while research employing MS for examining intact mRNA molecules (i.e., >200 nts) remains limited. Brophy et al. The study utilized IP-RP LC-TOF MS and CD-MS methods to characterize EPO mRNA (858 nts), Fluc mRNA (1909 nts), and Cas9 mRNA (4521 nts). Recent studies implemented native MS in positive mode to analyze mRNA with and without poly(A) tails demonstrating that mRNA lacking poly(A) tails show significantly reduced complexity. Native MS serves as an effective tool for distinguishing between n + 1/n + 2 transcripts and detecting dsRNA contaminants. Native MS provided precise detection of dsRNA variants with mass increments of +2.5 kDa and +5 kDa due to its high resolving power and non-denaturing conditions which then showed these variants as 3′-loopback dsRNA dependent on the T7 polymerase used during mRNA production. The results confirm high-resolution native MS as a powerful tool for mRNA characterization applications.
The innate immune system activation by residual DNA from IVT processes leads to adverse effects because this DNA serves as a common impurity. Researchers frequently use DNase treatment to eliminate residual DNA because this enzyme selectively breaks down DNA without harming mRNA. Physical filtration methods like microfiltration and ultrafiltration together with adsorption techniques using activated carbon or ion exchange resins are commonly applied for the removal of proteins and endotoxins from mRNA preparations. Double-stranded RNA serves as a major impurity which activates the innate immune system and diminishes mRNA therapy effectiveness. Detection and quantification of dsRNA can be achieved through gel electrophoresis and Northern blotting as well as quantitative PCR (qPCR). The application of RNase-free DNase and proteases for enzymatic digestion effectively removes dsRNA contamination from mRNA products. The quality of mRNA is negatively influenced by incomplete transcripts generated during the IVT process. HPLC methods including ion-pair reversed-phase HPLC (IP-RP HPLC) and SEC enable separation of full-length mRNA from shorter incomplete transcripts. Capillary electrophoresis techniques including CGE and capillary zone electrophoresis (CZE) deliver precise separation and analysis of mRNA which results in a final product without significant impurities.
It is essential to develop assays that evaluate the complete functionality of mRNA alongside physicochemical techniques which characterize its specific quality attributes. Functionality testing examines how well all analytical attributes of mRNA including capping efficiency and poly(A) tail formation work together to enable synthesized mRNA to translate into a functional protein. The main objective of these assays is to determine translation efficiency based on the production yield and quality of the resulting protein product. The techniques to determine mRNA translation efficiency vary widely from cell-free approaches using recombinant proteins through to advanced cell-based testing systems. Researchers find cell-free systems like in vitro translation assays appealing because they operate with simplicity. The assays function independently of the specific role of mRNA because they measure translation ability without assessing the encoded protein's subsequent activity. The system employs cellular lysates such as Rabbit Reticulocyte Lysate (RRL), Wheat Germ Extract (WGE), and HeLa Cell Lysate which provide all necessary components for translation within a cellular environment. These assays demonstrate greater biological relevance compared to fully recombinant systems including the PURE system. The efficiency of translation can be tracked by integrating luciferase reporter genes into the mRNA sequence and detecting luminescence which serves as a quantitative indicator of translation activity.
At BOC Sciences, we provide comprehensive analytical services to support the characterization, quality control, and regulatory readiness of mRNA-based therapeutics. Whether you're developing an mRNA vaccine, therapeutic protein, or gene editing system, our team offers the technical expertise and platform capabilities to ensure your product meets the highest quality standards.
We help you accurately assess RNA purity and detect degradation or impurities:
Ensuring that your sequence is correct and complete is essential:
Proper capping and polyadenylation are critical for translation and stability:
We detect and quantify critical impurities that may affect safety or efficacy:
We support ICH-compliant stability testing to understand product shelf life:
To correlate structure with biological activity:
From early research to IND-enabling studies, our analytical platforms and regulatory insight allow you to move forward with confidence. We tailor each testing strategy to your specific mRNA construct, ensuring thorough characterization, quality control, and batch-to-batch consistency.
Ready to discuss your project? Contact us to learn how we can accelerate your mRNA development through expert analytical support.
We employ UV-Vis spectroscopy for purity, capillary and gel electrophoresis for integrity, HPLC for capping and poly(A) tail analysis, and mass spectrometry for structural verification.
We use gel electrophoresis, dot blot, quantitative PCR, and Northern blotting to identify and quantify dsRNA contamination, ensuring high-quality mRNA free of immunogenic impurities.
The length and uniformity of the poly(A) tail directly affect mRNA stability and translational efficiency, making accurate assessment essential for quality control.
We assess IVT mRNA for sequence accuracy, integrity, capping efficiency, and impurity profiles using electrophoresis, HPLC, and mass spectrometry techniques.
We perform in vitro translation assays and reporter gene systems to measure protein expression and validate mRNA performance in functional applications.
Our advanced characterization enables optimization of sequence, structure, and modifications to design mRNA with enhanced stability, translation efficiency, and functional performance.
References