Early research stages demand rapid prototyping of mRNA constructs because this process offers multiple advantages which greatly improve therapeutic application development. Through prototyping researchers can rapidly evaluate and enhance mRNA designs before finalizing constructs which achieve optimal efficacy while maintaining stability and safety. Prototyping fulfills multiple essential purposes within the development process of mRNA-based therapeutic solutions. The initial function of prototyping is to validate the proof of concept by showing that mRNA constructs produce the targeted protein and achieve the desired therapeutic outcomes. Early validation plays a crucial role in research and development because it directs resource allocation toward the most promising candidates.
The prototyping process helps researchers detect and address potential problems before they become critical issues. The iterative testing and creation of multiple prototypes allows scientists to detect design flaws and enhance mRNA construct performance while optimizing sequence efficiency. The iterative method reduces risks that arise during late-stage changes by avoiding extensive time consumption and high costs. Prototyping allows scientists to investigate various design changes and enhancements. Researchers can discover new therapeutic targets as well as identify ideal protein production sequences and create stable and efficient mRNA constructs through this approach. The ability to quickly iterate and refine designs proves essential for mRNA therapeutics which undergo rapid advancements where new findings and innovations might significantly determine treatment outcomes.
The mature eukaryotic mRNA molecule consists of five key elements including the 5' cap structure (m7GpppN or m7Gp3N (N can be any nucleotide)), followed by the 5' untranslated region (5'UTR), an open reading frame (ORF), the 3' untranslated region (3'UTR), and it ends with a poly(A) tail. The basic structural domains of mRNA vaccines play a crucial role in their stability and effectiveness in triggering an immune response as well as their translation efficiency. The mRNA ORF establishes the fundamental sequence of the target protein along with RNA secondary structures that regulate translational effectiveness. Research has shown that mRNA coding regions which form secondary structures correspond to mRNAs with high expression levels. Ribosome binding requires the 5' UTR which acts as the site where the protein translation preinitiation complex forms. The main function of the 3′ UTR is to maintain mRNA stability. The stability of mRNA in eukaryotic cells is controlled by degradation signals located within their 3' UTR regions. Research has demonstrated that AU-rich areas within the 3' UTR assist in breaking down the poly (A) tail during mRNA degradation.
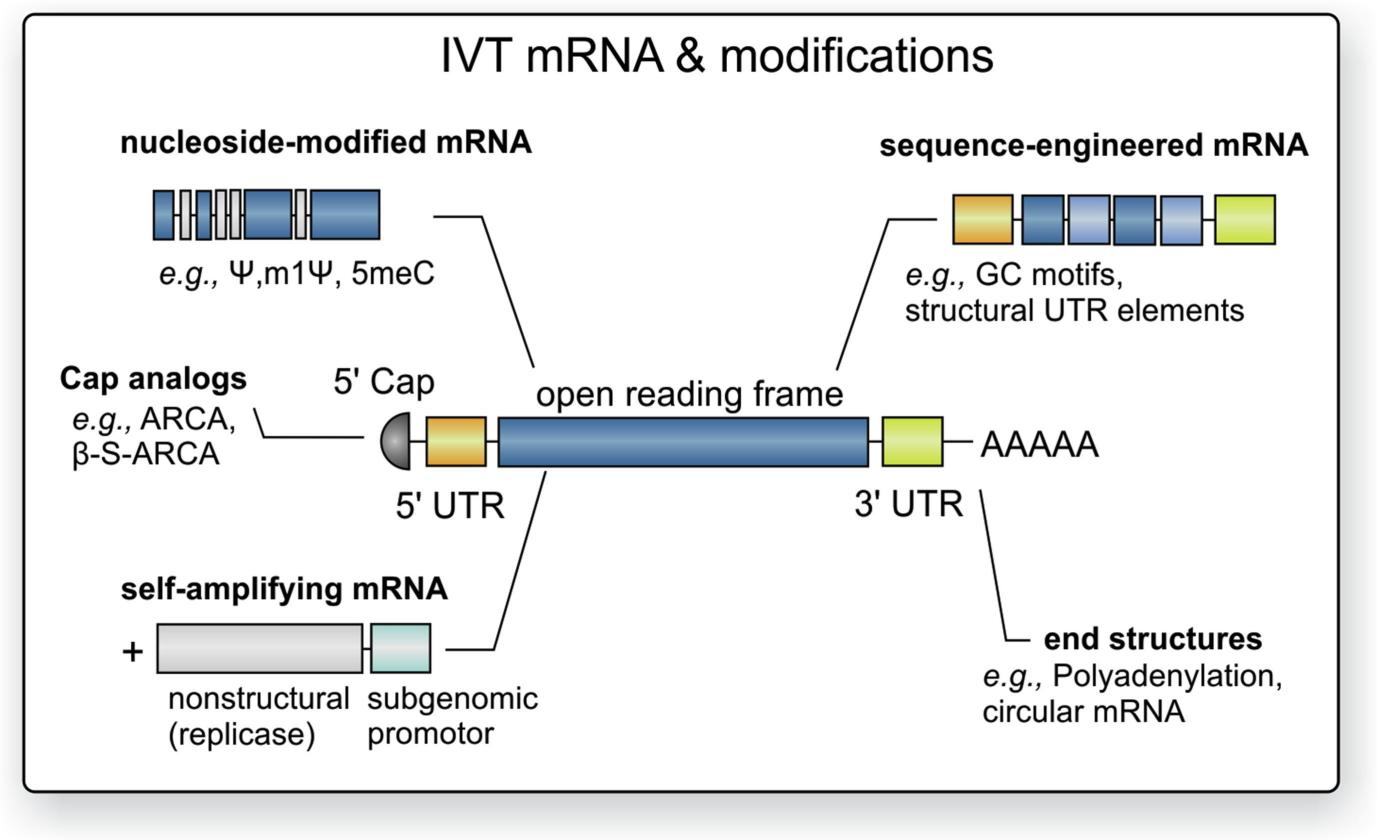 Fig. 1 Structural elements of in vitro transcribed (IVT) mRNA.1
Fig. 1 Structural elements of in vitro transcribed (IVT) mRNA.1
The ORF provides sequential information about the amino acids that compose a protein. Codon degeneracy allows multiple codons to encode the same amino acid so ORF design must choose codon combinations that produce the desired amino acid sequence while optimizing protein synthesis. Codon optimization involves choosing the best possible codon for each amino acid. One standard translation enhancement technique involves substituting rare codons with synonymous codons that are preferred by the cellular environment using host cell tRNA abundance information. Scientists measure synthetic synonymous codon usage bias through the codon adaptation index (CAI), which serves as an effective tool for predicting gene expression levels. The CAI measures the average codon usage frequency through geometric mean calculation for all codons in a given ORF that encodes a specific amino acid sequence. The relative codon frequency for any given codon is measured by dividing its observed frequency by the frequency of the most common synonymous codon from reference highly expressed genes. When ORFs consist exclusively of the most common codons for each amino acid the resulting CAI measures precisely 1.0. The approved mRNA vaccines BNT-162b2 and mRNA-1273 utilize optimized ORFs that achieve CAI values above 0.9. Experimental research has shown that choosing specific codons affects the duration mRNA remains active inside cells.
For 5' UTR: An artificially designed mRNA can achieve maximum antigen protein expression in target host cells through a 5′ capped UTR that ensures high ribosome loading efficiency. An optimal 5′ UTR can be determined by utilizing 5′ UTR sequences from human mRNAs that demonstrate high expression efficiencies within the specific target cell type. Under conditions of low cellular iron levels iron response elements (IREs) prevent ribosomal complex binding while upstream ORFs (uORFs) decrease translation efficiency through control over ribosomal preinitiation complex (PIC) entry into the ORF region. During the creation of mRNA vaccine sequences, only those regulatory components that enhance ribosome loading should be included in the 5′ UTR designs. The HIV-1 5′ UTR incorporates more than six functional structural motifs which control viral mRNA dimerization and splicing and also govern gene expression viral packaging and replication. The efficiency of gene expression in mRNA vaccines varies depending on the type of structural motifs present.
For 3' UTR: The 3′ UTR houses multiple essential regulatory elements which impact protein expression through their effect on mRNA stability inside cells despite being less vital than the 5′ UTR for mRNA expression regulation. AU-rich elements (AREs) which constitute 50 to 150 nucleotides and contain multiple copies of AUUUA motifs drive mRNA degradation as they interact with specialized ARE-binding proteins. MicroRNA (miRNA) response elements (MREs) represent another crucial element within gene regulation. miRNA binding to mRNA triggers translational repression which stops the mRNA from being translated to produce protein. The design of 3′ UTRs should avoid the inclusion of negative regulators like MREs which permits the use of specific human mRNAs whose 3′ UTRs lack MREs. The removal of MREs from 3′ UTRs risks disrupting other regulatory elements that are crucial for either increasing or decreasing protein translation. Human mRNA 3′ UTRs without MREs available today might fail to achieve high efficiency in protein translation. A thorough evaluation and optimization of all these factors is necessary.
The poly(A) tail stabilizes mRNA and enhances protein translation while translation efficiency increases with longer poly(A) tail lengths. The longevity of mRNA molecules depends critically on this factor. The template vector from which mRNA is transcribed contains the poly(A) tail information or alternatively the IVT mRNA undergoes enzymatic extension by recombinant poly(A)polymerase. The poly(A) polymerase enzyme introduces modified nucleotides into the poly(A) tail which blocks the action of poly(A)-specific nucleases responsible for deadenylation. The poly(A) tails of mammalian cells mRNA molecules measure approximately 250 nucleotides in length which decreases from the 3′ end to the 5′ end throughout their cytoplasmic existence. The synthesis of mRNA therapies benefits from poly(A) tails that measure around 100 nucleotides because the length of these tails influences mRNA stability through regulation of 3′ exonucleolytic degradation. Arbuthnot et al. Arbuthnot et al. proposed using iterative restriction digestion with type IIS enzymes followed by ligation and propagation to extend homopolymeric sequences to 100 bp on circular plasmids for cloning improvement. Researchers recommended using linear plasmids as mRNA synthesis templates to address existing limitations. The process of enzymatic polyadenylation can lead to RNA preparations that contain multiple RNA species characterized by inconsistent poly(A) tail lengths. RNA generated through In Vitro Transcription from a DNA template attains a predefined poly(A) tail length which enhances its clinical application suitability.
The global structure of ORFs works with local RNA structures including loops, hairpins, junctions, and pseudoknots to influence mRNA stability within cells alongside translation speed and precision. RNA local structures which develop from short sequence regions control mRNA in-cell degradation and ribosome movement speed along with potential translational frameshift stimulation. The instability of mRNA in cells arises from RNase-dependent RNA degradation mechanisms. The cellular RNase system includes two main types of enzymes: Exoribonucleases are enzymes that degrade RNA through the removal of terminal nucleotides from RNA's 5′ or 3′ ends while endoribonucleases cleave single-stranded or double-stranded RNA chains by recognizing specific RNA sequences and structural motifs. In order to prevent endoribonuclease activation researchers need to avoid certain RNA sequences and structural elements. Since single-stranded RNA (i.e. Endoribonucleases recognize loops as common structural elements in single-stranded RNA which makes mRNA sequences with reduced single-stranded regions beneficial for extending the mRNA lifespan within cells.
Researchers employ the Design-Build-Test (DBT) cycle as a primary method for creating mRNA constructs which are essential for therapeutic applications. Through iterative testing researchers quickly develop mRNA designs which they then refine and verify before optimizing the constructs for effectiveness, stability and safety. In the design phase researchers conceptualize and create plans for the mRNA construct. The design stage requires choosing the target protein and employing codon optimization for the ORF while selecting suitable 5′ and 3′ UTRs to improve both translation efficiency and mRNA stability. To predict mRNA design performance researchers can use advanced computational tools along with machine learning models to help choose the best sequences. During the build phase scientists work on physically constructing the mRNA construct. The process requires IVT for mRNA synthesis and includes steps for purification along with quality control measures to verify product purity and integrity. The build phase faces challenges related to achieving high-fidelity mRNA synthesis while scaling production capacity to fulfill demand. The test phase measures mRNA construct performance under both laboratory conditions and within living organisms. Researchers measure translation efficiency alongside protein expression levels and mRNA stability during this evaluation process. Researchers utilize advanced analytical methods including next-generation sequencing (NGS), mass spectrometry and multiple assays to evaluate the functionality and effectiveness of the mRNA construct. During the learn phase researchers analyze test phase data to extract insights for subsequent design iterations. Data analytics and machine learning enable the detection of patterns which helps optimize subsequent design iterations. Ongoing enhancement and development of the mRNA construct depends heavily on this phase.
Our mRNA constructs include 5' cap structures, optimized UTRs, codon-optimized ORFs, and poly(A) tails - all engineered to enhance stability and translation efficiency.
We replace rare codons with host-preferred synonymous codons to enhance translation efficiency, improve mRNA stability, and maximize protein expression levels.
5' UTRs regulate ribosome binding and initiation, while 3' UTRs influence mRNA stability and degradation rates - both critical for optimal expression.
We tailor poly(A) tail length (typically 100-150 nucleotides) to balance mRNA stability with translational efficiency for specific research applications.
We employ next-generation sequencing, mass spectrometry, in vitro translation assays, and stability testing to comprehensively evaluate construct performance.
Our iterative approach enables rapid prototyping, performance optimization, and data-driven refinement of mRNA constructs for research applications.
References