Developing mRNA for therapeutic use requires a complex approach to evaluate multiple factors which guarantee improved stability and expression during delivery. The development of mRNA therapeutics requires different approaches depending on whether they are used for vaccines or treatments of cancer and genetic disorders.
Stability and Modifications: Therapeutic mRNA applications achieve their effectiveness through molecule stability during the translation process that generates functional proteins. The mRNA sequence remains stable due to modifications with pseudouridine and 5-methylcytidine which concurrently lower immune system detection. When the 5′ and 3′ untranslated regions (UTRs) are optimized they enhance translation efficiency and extend mRNA half-life. Translation efficiency is determined by selecting cap structures like anti-reverse cap analogs (ARCA) and applying modern capping methods.
Delivery Systems: The delivery system selection determines an mRNA molecule's potential to successfully reach its target cells. The extensive adoption of lipid nanoparticles (LNPs) arises from their protective properties against mRNA degradation together with their support for cellular uptake. Refining the composition and formulation of LNPs results in improved delivery efficiency and diminished off-target effects. The development of mRNA vaccines improves both immune response and mRNA delivery to the cytosol through LNP surface modifications with polysaccharides or endosome escape molecules.
Personalized Therapies: Personalized medicine enables the customization of mRNA therapy designs for individual patients using their unique genetic and molecular profiles. Scientists can optimize codon usage and direct the therapy towards particular disease pathways through this approach. Whole-genome sequencing identifies neoantigens which can then be used to develop mRNA vaccine sequences delivered by LNPs for personalized cancer treatment. Through this strategy we achieve improved therapeutic effectiveness by making certain that the proteins produced by the therapy are precisely detected by the immune system.
Rapid Development and Clinical Translation: mRNA therapies develop rapidly and adjust well to different conditions which makes them powerful tools against emerging health threats. Precision medicine technologies such as next-generation sequencing and high-throughput screening enable swift identification of therapeutic targets while aiding in the creation of mRNA sequences that regulate gene expression. Scientific discoveries that can be quickly translated into clinical applications deliver new treatments to patients at a faster rate.
The development of mRNA vaccines depends heavily on antigen optimization which determines both safety and effectiveness. Multiple strategies are employed during the process to improve the antigen's immunogenicity and stability while reducing potential side effects. Identifying the target antigen that produces a strong immune response is the initial phase of antigen optimization. COVID-19 vaccine research centers on the SARS-CoV-2 virus spike protein (S protein) as the main target for immune response. The protein enables viral entry into host cells while serving as the main target for neutralizing antibodies. By inserting targeted mutations into the antigen sequence scientists can improve stability while boosting its ability to trigger an immune response. The mutation S-2P (K986P/V987P) maintains the spike protein in its pre-fusion state which results in higher immunogenicity. The S-2P mutation (K986P/V987P) which stabilizes the spike protein has been effectively implemented in COVID-19 vaccines. Altering antigen functional domains enhances how the immune system recognizes it. The spike protein's receptor-binding domain (RBD) serves as an essential target region for antibody interaction. The full-length spike protein provides effectiveness yet directing the immune response towards the RBD achieves strong protection. The antigen's expression and presentation improve when optimized signal peptides and transmembrane domains are integrated. Signal peptides guide proteins towards the cell surface or secretory path while transmembrane domains provide membrane stabilization for proteins. The process of iterative testing and refinement is essential for achieving continuous optimization. The process requires assessment of antigen variants in preclinical models followed by design refinement according to immunogenicity and safety data.
A crucial element of mRNA vaccine development is rapid prototyping because it enables swift iteration and enhancement of vaccine candidates. The development timeline is shortened through a multi-step process. Researchers use artificial intelligence and computational tools to quickly create and test potential antigens. Researchers use this process to discover new antigens for new pathogens and improve current antigens to achieve higher immunogenicity. After potential antigens are detected researchers conduct in vitro testing through cell cultures and in vivo testing with animal models. Researchers assess both the immunogenic potential and safety characteristics of the antigen through this step. Researchers analyze data from both in vitro and in vivo tests to improve antigen design. Through this iterative procedure scientists expedite antigen variant optimization to find the most effective versions. The LNP formulation undergoes optimization during this phase to guarantee effective mRNA delivery. Researchers evaluate multiple lipid compositions and formulations to achieve optimal stability and cellular uptake. After achieving optimization of the mRNA vaccine candidates they move forward into clinical trial phases. The rapid prototyping methodology enables swift adjustments and enhancements through clinical feedback which guarantees the vaccine's safety and effectiveness.
Neoantigen mRNA vaccines offer a revolutionary cancer immunotherapy method that activates a specific immune response by targeting individual genetic mutations found within a patient's tumor. These vaccines use encoding mechanisms for neoantigens that reflect each patient's unique tumor mutations while avoiding elements found in normal healthy cells. Researchers start the development process by acquiring a tumor biopsy from the patient which undergoes whole-exome and RNA sequencing to detect tumor-specific mutations. The patient-specific mRNA vaccine incorporates multiple neoantigens identified from the patient's tumor to initiate a targeted immune attack against cancer cells. Clinical trials have shown promising results. The combination of the personalized mRNA vaccine mRNA-4157 (V940) with the immune checkpoint inhibitor pembrolizumab achieved a marked reduction in recurrence risk among patients with high-risk resected melanoma. The autogene cevumeran vaccine (RO7198457/BNT122) currently undergoes multiple trials to test its effectiveness against different advanced or metastatic solid tumors such as bladder cancer. Despite the potential benefits, several challenges remain. Every patient's cancer presents unique characteristics that require customized vaccines which take extensive time and resources to create. Recent advances in computational algorithms together with next-generation sequencing technologies are improving the process for identifying and prioritizing neoantigens.
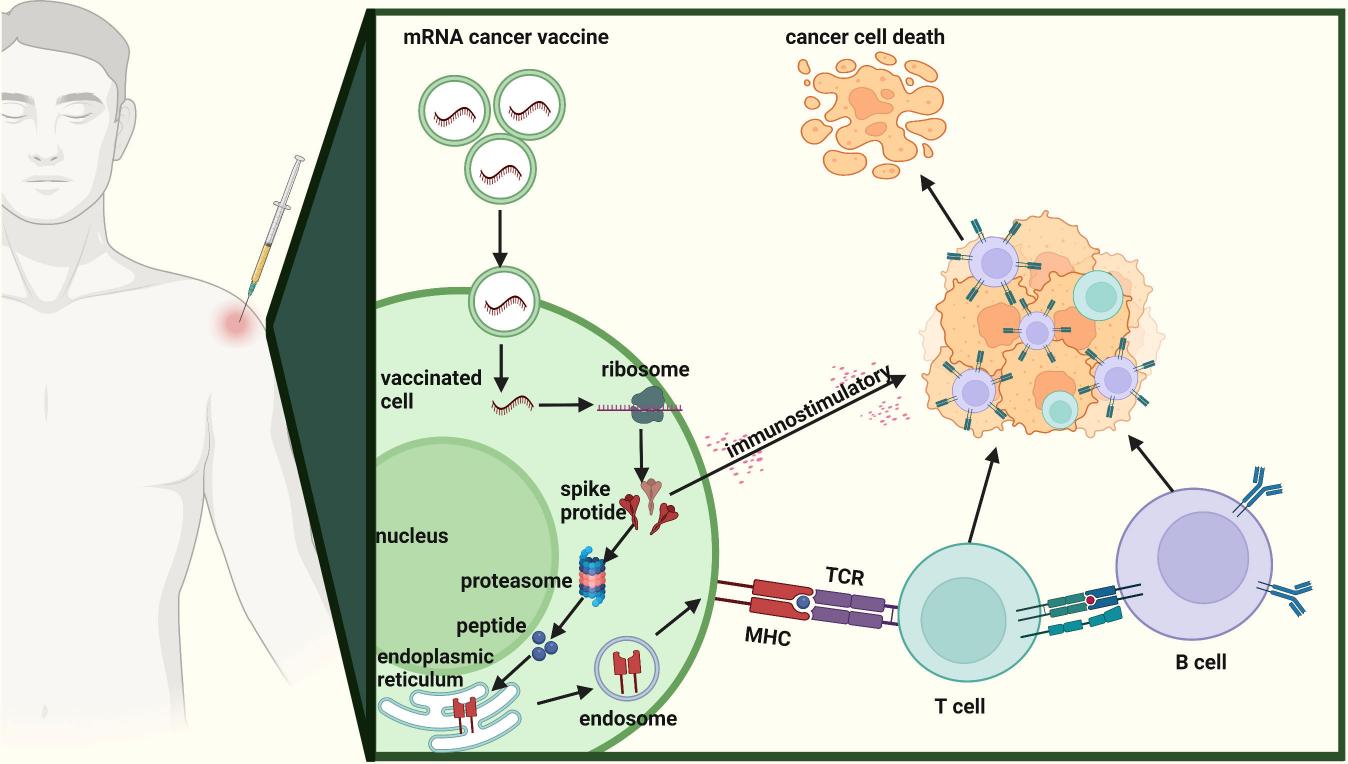 Fig.1 Mechanism of action of mRNA cancer vaccines.1
Fig.1 Mechanism of action of mRNA cancer vaccines.1
Immune modulators boost mRNA cancer vaccine efficacy through their ability to direct immune responses towards optimal antitumor activity. To establish an optimal immune environment for cancer treatment researchers administer mRNA vaccines together with cytokines and chemokines as well as additional immune-stimulating compounds. The effectiveness of immune checkpoint inhibitors and mRNA vaccines stems from their ability to block cancer cells from sending signals that help them stay undetected by the immune system. The KEYNOTE-942 trial showed improved recurrence-free survival (RFS) outcomes with the combination of personalized mRNA vaccine mRNA-4157 and pembrolizumab compared to pembrolizumab treatment by itself. The stimulation of the immune system can be directly accomplished through adjuvant application. Researchers demonstrated that TLR agonists including palmitic acid-modified TLR7/8 agonists improve the way mRNA-encoded antigens are presented and trigger stronger adaptive immune responses. The STING pathway activation through STING agonists leads to type I interferon production and these compounds have been investigated as mRNA vaccine adjuvants. The antitumor immunity of mRNA vaccines has been improved by using cytokine cocktails alongside adjuvants. Patients with advanced melanoma demonstrated promising tumor response rates when treated with dendritic cell-based mRNA vaccination enhanced by DCs electroporated with mRNA coding CD70, CD40 ligand and active TLR4.
Protein replacement therapy that relies on mRNA provides an effective method for treating diseases resulting from missing or malfunctioning proteins. In contrast to conventional protein replacement therapies which administer recombinant proteins directly to patients, mRNA therapies instruct the patient's cellular machinery to manufacture the necessary proteins. The scientific method described here demonstrates multiple benefits such as extended protein expression capabilities and diminished immunogenic responses while enabling the delivery of hard-to-reach intracellular proteins. In their research, Baba and colleagues demonstrated the use of mRNA therapy to supply neural cells with fully matured proteins and peptides to address neurological diseases. After intranasal administration in mouse models sustained protein expression was observed in nasal tissues for nearly two days which resulted in improved neurological recovery. Researchers treated hemophilia B in a mouse model using mRNA-LNP complexes which showed no activation of innate immune responses after multiple administrations. Despite these promising results, challenges remain. To achieve optimal therapeutic outcomes with mRNA-based protein replacement therapies researchers must solve both mRNA instability and immune activation issues. The development of modified mRNA molecules and advanced delivery systems like LNPs represents essential progress toward solving stability and immune response issues while delivering therapeutic mRNA effectively.
The mRNA technology field has forged new paths with mRNA-based gene editing which provides an effective method for correcting genetic mutations and treating genetic diseases. This method uses mRNA to transmit gene-editing elements like CRISPR-Cas9 which then modifies the host genome. Gene editing with mRNA offers multiple benefits compared to conventional techniques by enabling temporary expression of editing molecules while reducing off-target effects and preventing permanent genetic material integration. Scientists have successfully applied mRNA encoding CRISPR-Cas9 in animal models to perform in vivo gene editing which shows promise for metabolic and genetic disease therapies. By using transient editing components scientists reduce both the duration of immune system activation and the chance of off-target effects which are typical complications found in gene-editing treatments. mRNA delivery systems enable the transport of other gene-editing mechanisms like TALENs in addition to CRISPR-Cas9. Creating effective delivery systems like LNPs stands as an essential requirement for the practical application of mRNA-based gene editing in medical practices. Research and clinical trials persist in investigating the best delivery techniques while optimizing mRNA construct designs to improve safety and efficacy in gene-editing treatments.
At BOC Sciences, we understand that each therapeutic area presents unique challenges and requirements when it comes to mRNA design. That's why our services are fully customizable based on your application-whether you're developing a preventive vaccine, a cancer immunotherapy, or a therapeutic protein replacement strategy.
We offer design and optimization solutions for rapid, scalable vaccine development:
For cancer vaccines and mRNA-based immunotherapies, precision and immunogenicity are key:
When therapeutic mRNA is used to restore or supplement a deficient protein:
For gene editing payloads, precision, durability, and controlled kinetics are essential:
By aligning mRNA sequence engineering with therapeutic goals, we help you navigate the complexities of each application with speed and confidence. Our team combines deep domain knowledge with robust technical platforms to support your journey from concept to clinic.
Need help designing your next mRNA therapeutic? Contact us today to explore how we can accelerate your development program.
We tailor mRNA sequences based on specific application requirements, including sequence optimization, UTR selection, capping strategies, and modifications to enhance stability and translation efficiency.
We implement chemical modifications like pseudouridine and 5-methylcytidine, optimize 5'/3'UTRs, and perform codon optimization to maintain mRNA integrity and extend functional half-life.
We formulate lipid nanoparticles with protective compositions, enhanced cellular uptake properties, and surface modifications to support targeted mRNA delivery.
We use sequencing data and computational analysis to design mRNA sequences encoding neoantigens or customized proteins, supporting precision in experimental applications.
We employ in vitro translation assays, reporter gene systems, and cell-based expression analyses to assess protein production and translation efficiency.
We optimize cap structures, poly(A) tail lengths, and UTR sequences to enhance ribosome binding, increase translation output, and reduce degradation for consistent performance.
References