Researchers use IVT as a technique to create RNA molecules from DNA templates outside of living cells in laboratory settings. This technique duplicates how cells perform transcription by using RNA polymerase to create RNA molecules from DNA templates. The synthesis of mRNA for medical treatments such as vaccines and gene therapies depends critically on the IVT process. The production of synthetic mRNA requires a cell-free enzymatic IVT reaction that uses a DNA template to direct the sequence formation along with free nucleoside triphosphate (NTP) monomers and DNA-dependent RNA polymerase which transcribes mRNA from the template in an optimized buffer environment. The yield and quality of the final product depend heavily on the components and their stoichiometric ratios in the reaction mixture and the physical parameters of the process including duration, pH and temperature as well as the design of the mRNA encoded on the template. The performance of the IVT process directly depends on how the encoded mRNA is designed including its length and the sequence of untranslated regions (UTR) and open reading frame along with the number of repetitive sequences and homogenic parts and the templated poly(A) tail.
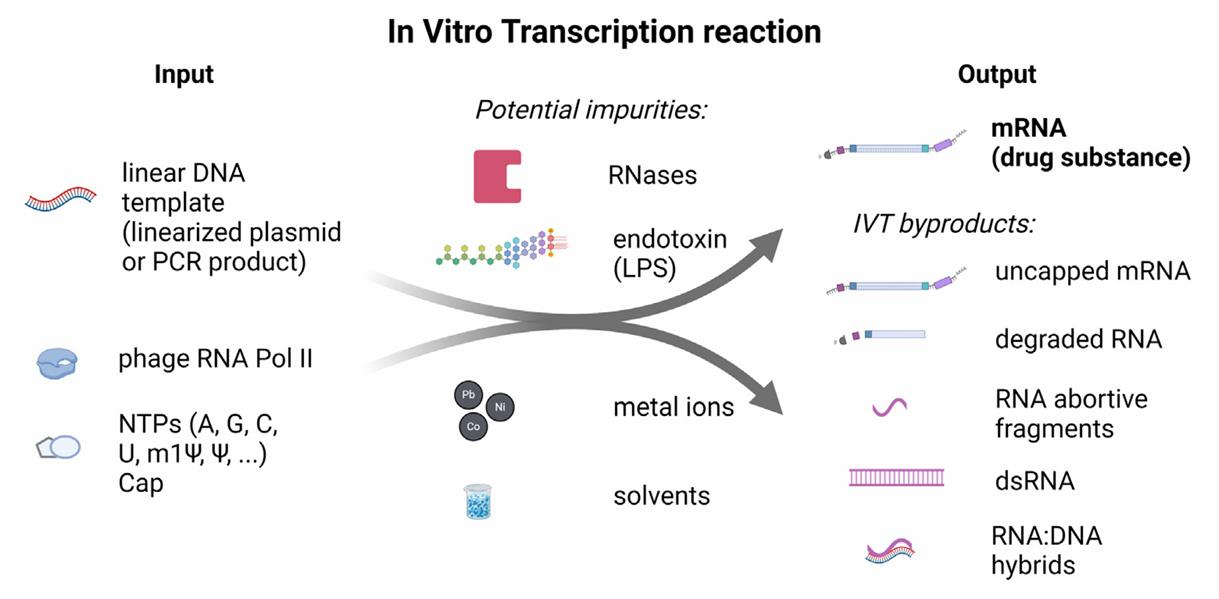 Fig.1 In vitro transcription reaction input and output components and potential impurities.1
Fig.1 In vitro transcription reaction input and output components and potential impurities.1
The efficiency of IVT depends on the quality of the DNA template used. The RNA polymerase uses the DNA template as a guide to produce mRNA. Synthesized mRNA requires high yields and purity which can only be achieved with high-quality DNA templates. To achieve efficient transcription the DNA template needs to be purified from contaminants and converted to a linear form. Linearized templates establish clear starting and stopping points for transcription which enable the generation of complete mRNA molecules. Residual plasmid DNA together with other contaminants may obstruct transcription and result in incomplete transcripts. Efficient transcription initiation requires the presence of a strong promoter sequence such as T7, T3 or SP6. To initiate effective transcription RNA polymerase needs to bind to the promoter sequence which must be properly placed upstream of the gene of interest. The amount of DNA template used in the IVT reaction plays a key role in determining the resulting mRNA yield. The optimal DNA template concentration for in vitro transcription changes based on the construct being used and specific reaction conditions but generally results in increased mRNA yields when template concentrations are higher. Excessive template amounts can cause double-stranded RNA (dsRNA) production which activates immune responses during therapeutic use.
RNA polymerase enzyme concentration during the IVT reaction remains an essential factor to control. The RNA polymerase enzyme enables RNA synthesis from a DNA template and the reaction's transcription efficiency depends directly on the enzyme's concentration. Increasing the RNA polymerase concentration during transcription enhances mRNA production efficiency for extended transcripts. Increasing enzyme concentration reaches a threshold where it stops enhancing yield but begins to raise costs. The performance level of RNA polymerase together with its functional activity play critical roles. The performance of enzymes varies between suppliers because of efficiency differences making it necessary to use only high-quality purified enzymes for reliable results.
Optimal concentrations and nucleotide ratios within the IVT reaction mixture are essential to produce high amounts of mRNA. Each nucleotide concentration requires optimization so that RNA polymerase possesses adequate building blocks for RNA synthesis. Standard nucleotide concentrations range from 1 to 2 mM for each type yet these levels may change based on the specific conditions of the reaction. The balance between magnesium ions (Mg2+) and nucleotides represents a crucial parameter. The enzymatic function of RNA polymerase requires Mg2+ ions and efficient transcription depends on maintaining the correct ratio between Mg2+ ions and nucleotides. Insufficient Mg2+ levels will decrease enzyme performance but excessive levels lead to dsRNA production. Synthesized mRNA displays enhanced stability and diminished immunogenicity when modified nucleotides like N1-methylpseudouridine (m1ψ) are implemented. RNA polymerase incorporates modified nucleotides effectively while maintaining the expected production yield.
Both the reaction time duration and the reaction temperature for IVT reactions play critical roles in determining the efficiency of mRNA synthesis. The IVT reaction process usually requires several hours to reach completion. Extended reaction times typically produce higher mRNA yields but eventually reach a maximum point beyond which further extension brings no additional yield benefits. An IVT reaction usually runs for 2-4 hours yet can be modified according to the specific molecular construct and the targeted yield. The IVT reaction occurs at 37°C because it represents the optimal temperature for RNA polymerase enzyme activity. Research has indicated that mRNA quality can benefit from temperature conditions that are slightly below or above standard levels. A stable temperature during the reaction is essential for consistent transcription while reducing production of truncated transcripts.
IVT experiments often experience low yield and poor quality because of degraded DNA templates. For transcription to occur efficiently the DNA template must remain intact because damage or fragmentation interferes with RNA polymerase's capacity to produce complete mRNA molecules. The presence of low mRNA yield along with incomplete or fragmented transcripts is indicative of template degradation and these symptoms become evident when analyzed via agarose gel. The template degradation process can result from repeated cycles of freezing and thawing along with incomplete enzymatic digestion of plasmid DNA and mechanical shearing during excessive pipetting. The use of high-quality DNA which is either freshly prepared or correctly stored proves essential in minimizing DNA degradation according to these recommendations. Storing DNA in small aliquots minimizes freeze-thaw cycles to maintain template integrity. The quality of the DNA template must be confirmed through gel analysis before starting IVT to ensure its integrity. Complete digestion of plasmid DNA and enzyme removal prior to template use for IVT helps prevent degradation. Researchers who manage these factors effectively can boost both the efficiency and quality of their IVT reactions to produce more intact and functional mRNA.
RNase contamination remains a widespread challenge in IVT experiments because even minimal RNase exposure leads to rapid RNA degradation which produces transcripts of low quantity and poor quality. RNA degradation and low yields of mRNA serve as primary indicators of RNase contamination because degraded RNA forms a smear pattern instead of distinct bands on agarose gels. Multiple factors contribute to RNase contamination including contaminated reagents and water buffers together with RNase present in the laboratory environment and on surfaces, equipment and pipettes. RNase can enter experiments through human contact including handling with hands or clothing. Using certified RNase-free reagents, water, and buffers during the IVT reaction is essential for reducing RNase contamination. You can lower contamination risks by creating an RNA-specific workspace that remains RNase-free and utilizing RNase-free products like pipette tips and tubes. It is essential to perform routine cleaning of lab surfaces and equipment with solutions that eliminate RNase. Researchers can reduce contamination risks by wearing RNase-free gloves and lab coats while avoiding direct contact with reagents or equipment. Researchers must utilize RNase-free water throughout IVT procedure steps such as dilutions and washes to maintain contamination-free conditions. Frequent testing for RNase contamination in reagents and equipment through RNase alert kits or alternative detection methods helps in identifying and resolving contamination problems quickly. Through the application of strict procedures researchers establish an RNase-free area which improves both IVT reaction performance and product quality to achieve increased production of complete functional mRNA.
Selecting appropriate enzymes and reagents during IVT is essential because these choices determine the final yield, quality, and functionality of the produced mRNA. The efficiency and reproducibility of IVT experiments improve significantly when proper enzymes and reagents are selected.
Selecting an appropriate RNA polymerase determines transcription efficiency. T7 RNA polymerase together with T3 and SP6 RNA polymerases represent the most frequently utilized enzymes in transcription processes. Every enzyme requires distinct optimal conditions along with particular promoter sequences for effective performance. Researchers often select T7 RNA polymerase because it exhibits both high activity levels and precise specificity toward the T7 promoter. The experimental requirements of IVT determine which polymerase to use based on the target yield and transcript length. The results of experiments require high-quality and pure RNA polymerase for consistency. Full-length transcript production becomes more efficient when high-purity enzymes reduce background interference and contamination remains low. Researchers should select enzymes from trustworthy suppliers who supply comprehensive specifications and quality assurance data. The amount of RNA polymerase used in the IVT reaction has a major impact on the production levels of mRNA. As enzyme concentration increases the yield goes up to a certain point after which additional enzyme does not produce proportional increases in yield. Too much enzyme results in double-stranded RNA (dsRNA) production that initiates immune responses during therapeutic treatments. Titration experiments should be used to optimize enzyme concentration for the best results.
High-purity nucleotides are essential for efficient transcription. Nucleotide impurities can prevent RNA polymerase activity or result in the creation of incomplete transcripts. It is necessary to optimize nucleotide ratios to achieve balanced transcription incorporation. The standard nucleotide concentration ranges between 1-2 mM but can be adjusted based on the reaction conditions. The RNA polymerase requires the IVT reaction buffer to sustain ideal pH levels and ionic conditions. Enzymes need Mg2+ to function properly and researchers must experimentally determine the best Mg2+ concentration. The activity of enzymes may need other co-factors including DTT (dithiothreitol) for proper function. Incorporating modified nucleotides like pseudouridine (ψ) or N1-methylpseudouridine (m1ψ) into mRNA synthesis improves its stability while lowering its immunogenicity. RNA polymerase can incorporate these modifications into synthesized RNA efficiently without lowering the yield significantly. All substances involved in the IVT process must be RNase-free to ensure RNA does not degrade. The necessary components for the procedure include RNase-free water, buffers as well as consumables like pipette tips and tubes. The integrity of the synthesized RNA can only be preserved with RNase-free reagents. The DNA template must demonstrate superior quality and structural integrity. The production of RNA requires linearized templates that contain a robust promoter sequence for effective transcription. The template requires a contaminant-free state and appropriate storage conditions to minimize any degradation.
At BOC Sciences, we offer a streamlined and fully optimized in vitro transcription (IVT) workflow designed to deliver high-yield, high-purity mRNA tailored to your specific research or therapeutic needs. Our process is built around precision, flexibility, and scalability-whether you're developing mRNA for vaccines, gene therapies, or protein replacement strategies.
We begin with the preparation or validation of high-quality linearized DNA templates:
We also offer codon optimization, UTR engineering, and sequence modifications to maximize transcription efficiency and downstream mRNA performance.
Our IVT process uses optimized reagent ratios and proprietary reaction conditions to maximize transcript yield and minimize impurities:
We support standard and modified nucleotide incorporation (e.g., pseudouridine, 5mC) to enhance mRNA stability and reduce immunogenicity.
To improve product quality and reduce contaminants:
Each step is tightly controlled to ensure lot-to-lot consistency.
We apply advanced purification methods to remove impurities such as dsRNA, short transcripts, and residual enzymes:
Purification workflows are selected based on your desired application and regulatory requirements.
All final mRNA products undergo rigorous QC to ensure quality, reproducibility, and compliance:
mRNA yield and purity quantification
With our optimized IVT workflow, you receive high-quality, transcription-ready mRNA with the yield, purity, and modifications necessary for success in research or therapeutic development.
From template to transcript, we deliver excellence at every step.
Key factors include DNA template quality, RNA polymerase concentration, nucleotide ratios, reaction duration, and temperature optimization, all of which we systematically optimize for maximum yield.
We employ rigorous template preparation, optimized enzyme systems, balanced nucleotide ratios, and advanced purification methods to produce high-integrity mRNA transcripts.
We utilize silica column purification, HPLC/FPLC techniques, and specialized dsRNA depletion protocols to eliminate impurities and ensure transcript purity.
Yes, we efficiently incorporate modified nucleotides including pseudouridine and 5-methylcytidine to enhance mRNA stability and reduce immunogenicity in research applications.
We perform comprehensive template analysis including promoter verification, linearization confirmation, and purity assessment to ensure optimal transcription efficiency.
Our QC includes yield quantification, capillary electrophoresis, dsRNA detection, capping efficiency analysis, and functional validation for batch-to-batch consistency.
References