The use of mRNA as vaccines and drugs to prevent, diagnose and treat certain diseases is gradually becoming the focus of pharmaceutical companies. mRNA-based therapy, in short, is to introduce chemically modified mRNA molecules into the cytoplasm, and use the nucleotides in the cytoplasm for transcription and expression to produce the proteins needed by the body.
The mRNA vaccine uses the target/antigen to encode mRNA, and allows cells to take up and express the encoded antigen through a specific delivery system, thereby causing humoral and cell-mediated immune responses. After vaccination, the mRNA vaccine encoding the spike protein of the new coronavirus encapsulated in lipid nanoparticles enters the cell and synthesizes the protein in the ribosome. The protein is either broken down into smaller fragments (polypeptides) by the cellular proteasome, or transported to the outside of the cell through the Golgi apparatus. The antigen polypeptide fragments enzymatically digested in the cell form a complex with the major histocompatibility complex (MHC) class I protein, which expresses the antigen on the surface of the presenting cell and is recognized by CD8+ T cells to induce cell-mediated immunity (Fig.1 left side). On the other hand, the extracellular spike protein can be swallowed, pinocytosed and broken down into smaller polypeptide fragments by different immune cells, forming complexes with MHC class II proteins, expressing antigens on the surface of presenting cells, and being CD4+ T cells recognize and promote B cells to produce antigen-specific antibodies. (Fig.1 right side)
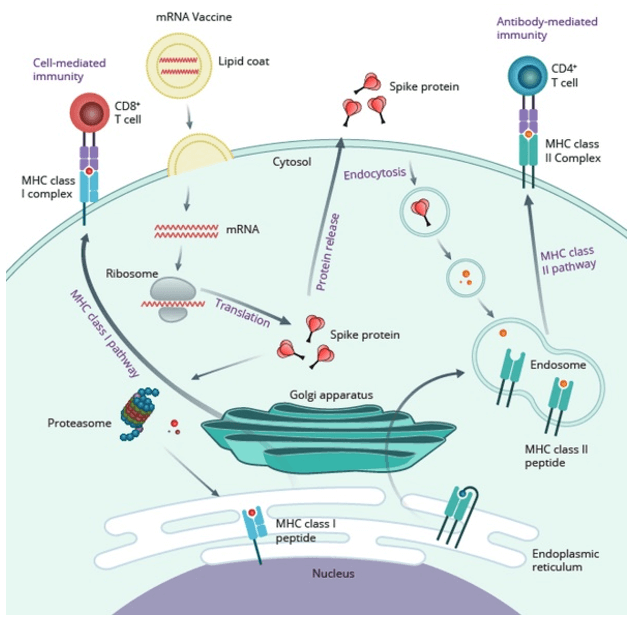 Figure 1: Mechanism of action of mRNA vaccine (Itziar Gómez-Aguado. 2020).
Figure 1: Mechanism of action of mRNA vaccine (Itziar Gómez-Aguado. 2020).
Currently, mRNA construction strategies have two directions: nonamplifying mRNA and self-replicating mRNA (SAM) (Fig. 2). RNA is easily degraded in the external environment, which affects translation and expression efficiency. In order to stabilize its structure and improve expression efficiency, non-replicating mRNA has been sequenced with the structure of eukaryotic mRNA as a reference. SAM is to chimeric the coding sequence to a vector with RNA-dependent RNA polymerase (RdRP), and increase the expression efficiency by copying its own mRNA by RdRP. The elements of non-replicating mRNA include 5'cap, 3'Poly(A) tail, untranslated region (UTR), and coding sequence (Fig. 3).
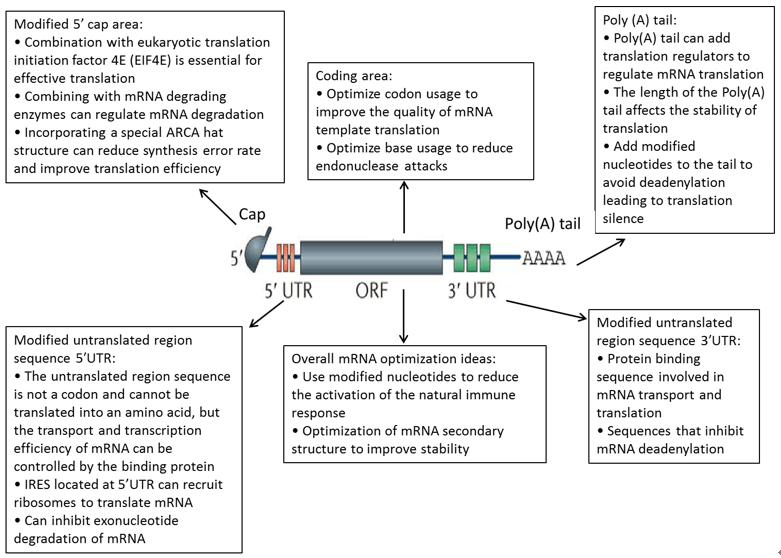 Figure 2. mRNA drug structure
Figure 2. mRNA drug structure
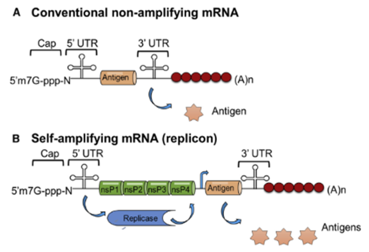 Figure 3. Structural composition of non-amplifying(A) and self-amplifying(B) mRNAs (Kowalski P S. 2019).
Figure 3. Structural composition of non-amplifying(A) and self-amplifying(B) mRNAs (Kowalski P S. 2019).
Regarding the characteristics of mRNA not being stable enough and having a short half-life, researchers have also found a variety of methods to enhance the stability of mRNA and prolong the half-life. For example:
Non-amplifying mRNA directly encodes target proteins, while self-amplifying mRNA includes RNA polymerase for intracellular replication, significantly enhancing protein expression levels and duration.
We optimize mRNA stability using anti-reverse-cap analogs, poly(A) tail engineering, stable UTR sequences, and nucleotide modifications to reduce degradation and improve expression.
Codon optimization replaces rare codons with synonymous high-frequency codons to enhance translation efficiency, mRNA stability, and overall protein expression levels.
UTRs critically regulate mRNA stability and translation; 5' UTR affects ribosome binding while 3' UTR influences intracellular localization and half-life.
Our optimized lipid nanoparticle systems protect mRNA from degradation, enhance cellular uptake, and ensure proper intracellular release for maximal protein expression.
We incorporate chemically modified nucleotides and optimized in vitro transcription to minimize immune recognition while maintaining high translation efficiency.
References