To create an effective mRNA therapy researchers must carefully evaluate multiple essential components and strategies to enhance mRNA stability as well as its translation efficiency and functional performance.
The 5′ end of an mRNA transcript receives a modified guanine nucleotide shortly after transcription which creates the 5′ cap. The mRNA is joined to the 7-methylguanosine cap by a unique 5′-5′ triphosphate linkage. The mRNA molecule maintains stability and functionality through the attached cap structure. The cap structure protects the mRNA transcript against exonuclease destruction and aids ribosomal identification during translation initiation while also facilitating nuclear export control. Specialized capping enzymes or cap analogs enable optimization of the mRNA cap structure which enhances its functionality.
The 5′ UTR which precedes the coding sequence functions in various ways to control gene expression. Specific proteins and small molecules interact with regulatory elements in this region to manage translation control. The ability of ribosomes to access mRNA molecules is determined by the secondary structures of the mRNA which feature hairpins and loops. uORFs control how ribosomes move toward the main coding sequence of mRNA. The manipulation of the 5′ UTR leads to optimal translation efficiency and precise ribosome positioning.
The mRNA sequence responsible for encoding the target protein is known as the ORF. Researchers enhance ORF performance by applying codon optimization which swaps rare codons for common synonymous codons to improve translation efficiency. Modifications to the ORF enable removal of sequences that have potential to trigger immune responses or destabilize mRNA. The selection of codons impacts both how proteins fold and their overall functionality.
The mRNA molecule's 3′ UTR reaches its terminus and functions as a crucial component of post-transcriptional control mechanisms. The 3′ UTR region hosts specific sequences that engage with RNA-binding proteins and microRNAs which affect the stability and degradation of mRNA. Localization signals within the 3′ UTR guide mRNA to distinct cellular compartments. Through 3′ UTR optimization researchers can increase mRNA stability and direct its correct localization inside the cell.
The mRNA receives its poly(A) tail at the 3′ end which functions as a key element for maintaining mRNA stability and enhancing translation efficiency. The mRNA tail contains repeated adenine nucleotides and its length can differ. The length of poly(A) tails usually determines mRNA stability and translation efficiency with longer tails providing better performance in both areas. Poly(A)-binding proteins (PABPs) interact with the poly(A) tail to enhance mRNA stability and improve ribosome loading.
Using lipid nanoparticles (LNPs) to formulate mRNA demonstrates a strong approach to improve both stability and delivery efficiency of mRNA molecules. Lipid nanoparticles shield mRNA from enzymatic breakdown while also promoting its entry into cells. The stability and effectiveness of mRNA delivery depend heavily on the lipid composition and ratios used in LNPs. Therapeutic effectiveness of mRNA therapies can be enhanced by optimizing LNP formulation.
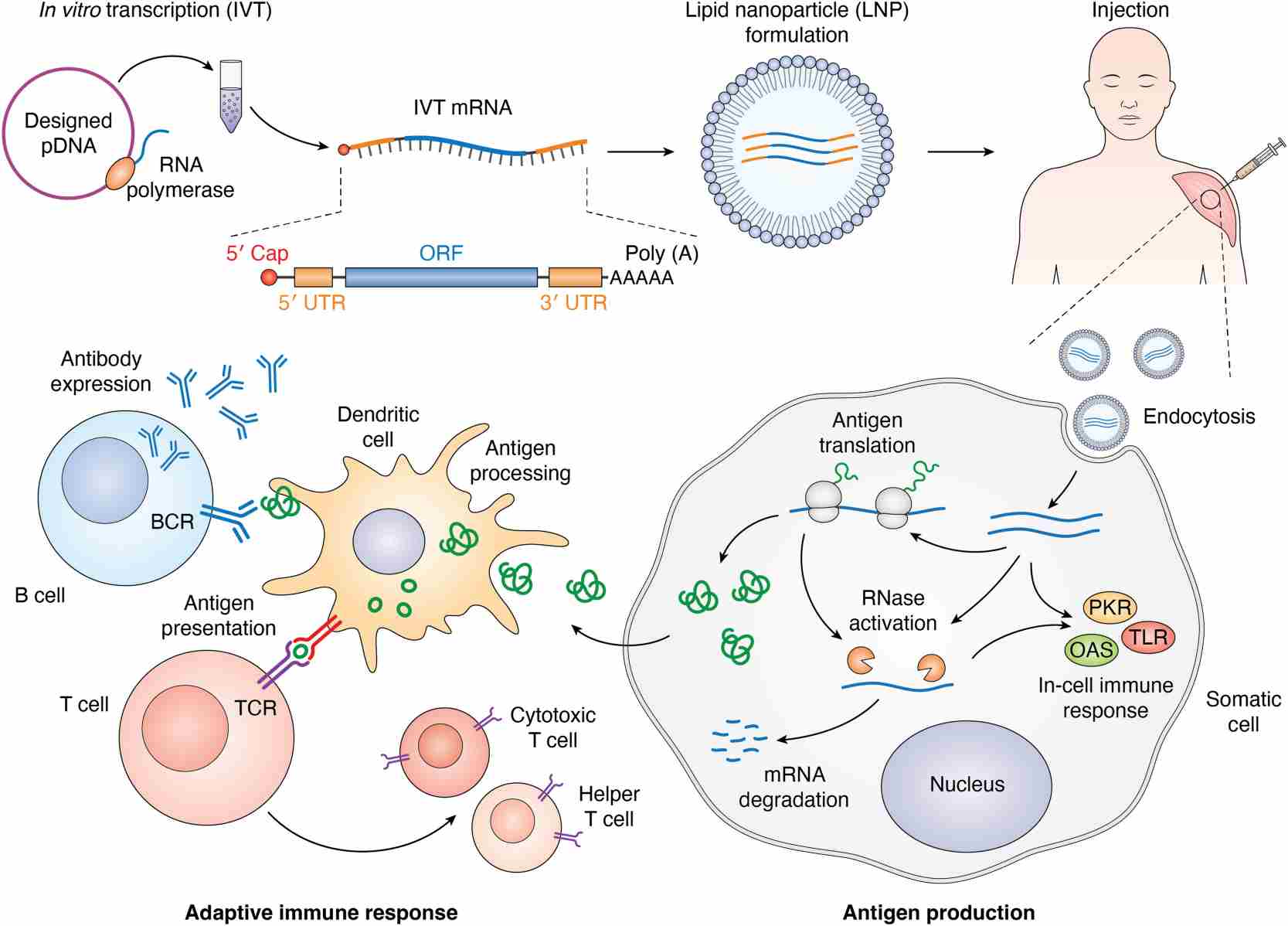 Fig.1 Simplified mRNA vaccine manufacturing procedure and mechanism of action.1
Fig.1 Simplified mRNA vaccine manufacturing procedure and mechanism of action.1
The design of mRNA ORF requires codon optimization to improve translation efficiency while maximizing protein production. The gene of interest undergoes DNA sequence modification to incorporate codons preferred by the target organism while preserving the protein's amino acid sequence. The efficiency of translation can be improved through codon optimization since it ensures that mRNA translation occurs via the most plentiful tRNAs present in the host cell. The usage of this technique becomes crucial when expressing genes in heterologous systems due to differences in codon usage bias between the host organism and the original organism. Researchers can utilize codon optimization tools to reach the ideal Codon Adaptation Index (CAI) for their chosen gene across different organisms. Researchers can prevent restriction enzyme cleavage sites through these tools while they optimize their target sequences. Codon optimization enhances mRNA stability through reduced uridine content which decreases Toll-like receptor activation and lowers mRNA immunogenicity.
The reduction of mRNA immunogenicity requires the avoidance of immunogenic motifs especially when designing therapeutic mRNA applications. Immunogenic motifs represent specific sequences that initiate immune responses which can result in the breakdown of mRNA or negative outcomes in the host organism. To prevent these motifs from activating immune responses researchers can decrease the presence of CpG motifs which are recognized by the innate immune system. Reducing the immunogenicity of mRNA can be achieved through optimization of GC content and avoidance of repetitive sequences. There exist tools and algorithms which help detect and adjust these motifs throughout the design phase to produce mRNA sequences that decrease the chance of activating an immune response.
The 5′UTR serves as a key regulator of mRNA translation efficiency. Enhancing protein expression levels requires optimizing the 5′ UTR sequence. A robust Kozak consensus sequence located near the start codon plays a key role in enabling ribosome attachment and initiating translation. The sequence enables ribosome detection of the start codon to initiate translation. Upstream stop codons along with secondary structures and G-quadruplexes within the 5′ UTR function as translation repressors. A 5′ UTR with a strong Kozak sequence that remains minimal should be used to prevent interference from repressive elements. Research indicates that translation efficiency experiences a slight increase when the 5′ end of the UTR contains a T-rich motif. The integration of this motif into the 5′ UTR design achieves increased expression levels. Extended 5′ UTR sequences can bring about unforeseen suppressive elements which decrease translation efficiency. Therefore, shorter 5′ UTRs are generally preferred. Deep learning models and advanced optimization algorithms enable researchers to create 5′ UTRs that enhance protein production. The application of these methods produces varied sequences which bypass typical problems while enhancing translation performance.
The 3′UTR serves as a key component that maintains mRNA stability and thereby determines mRNA longevity. AREs added to the 3′ UTR work as binding sites for RNA-binding proteins such as HuR which stabilizes mRNA by preventing its degradation. The presence of these proteins protects mRNA molecules from degradation mechanisms which extends their period of functionality. Stability of mRNAs increases when 3′ UTRs from naturally occurring stable mRNAs are employed. Specific 3′ UTRs of p53 target genes demonstrate unique stability profiles that enable precise tuning of mRNA stability. The stability of mRNA can be increased by eliminating sequences that trigger degradation such as those binding specific microRNAs. The desired outcome is reached by designing sequences meticulously and optimizing them. The stability of mRNA molecules can be affected by both the length and composition of their poly(A) tails. The poly(A) tail length correlates with mRNA stability since longer tails tend to be more stable yet the ideal length should be tailored to fit specific functional needs. Different configurations of 5′ and 3′ UTRs need to be tested to determine the best combinations for particular therapeutic applications. Research indicates that specific 5′ UTRs like those from complement factor 3 (C3) and cytochrome p4502E1 (CYP2E1) improve protein expression levels across multiple 3′ UTR combinations.
Signal peptides function as vital guides for delivering proteins to designated cellular compartments and improving secretion mechanisms and they play a key role in mRNA-based therapies and vaccine developments. Peptides with short amino acid sequences direct the ribosome-mRNA complex to the endoplasmic reticulum (ER) where they facilitate the transfer of the translated target protein into the ER lumen during translation. The mechanism serves dual functions by ensuring correct protein folding and post-translational modifications while simultaneously increasing protein secretion efficiency. The use of signal peptides in mRNA constructs boosts both the production yield and stability of therapeutic proteins. A study examined multiple signal peptides to determine their effectiveness at enabling the extracellular secretion of NanoLuc luciferase (Nluc) across various cell lines. Research demonstrated that the cystatin S signal peptide achieved the highest levels of Nluc secretion across every cell type examined. Optimizing signal peptides shows potential to enhance mRNA vaccine immunogenicity through better antigen expression and secretion and improved presentation.
Functional and structural modifications can be achieved through the incorporation of tags into mRNA constructs. Endogenous proteins can receive chemical tags through RNA base editing methods along with the site-specific addition of non-canonical amino acids. The technique enables proteins to be tagged with tiny chemical probes which facilitates the study of protein dynamics and interactions within living cells. Protein targeting to specific cellular locations can be achieved through modifications like GPI anchor addition or the insertion of transmembrane domains besides chemical tagging. During malaria transmission-blocking vaccine development researchers explored mRNA constructs that contain signal peptides alongside GPI anchors and transmembrane domains to boost target antigen functional expression. The effectiveness of mRNA-based vaccines increases through modifications that guarantee the proper localization and presentation of expressed proteins to the immune system.
At BOC Sciences, we provide comprehensive, end-to-end support for custom mRNA design-transforming your target gene sequence into a high-performing, translatable mRNA molecule. Whether you're developing a vaccine, therapeutic protein, or gene editing tool, our services are tailored to enhance expression, stability, and biological efficacy from the ground up.
We start by engineering a sequence that maximizes expression in your target system:
Proper mRNA processing elements are critical for translation and stability:
We offer a wide range of chemical modifications to improve mRNA behavior:
We employ advanced algorithms to ensure optimal secondary structure and safety:
We help prepare for scalable in vitro transcription:
Ensure your designed mRNA delivers the expected biological output:
From bioinformatic analysis to expression-ready, highly stable mRNA constructs, our integrated services ensure scientific precision and regulatory foresight. Partner with us to streamline your mRNA project with a fully customized and quality-driven approach.
We optimize codon usage, GC content, secondary structures, and regulatory elements including 5'and 3'UTRs to enhance translation efficiency and stability.
Optimized 5'and 3'UTRs regulate ribosomal binding, translation efficiency, and mRNA stability, ensuring consistent functional performance in various applications.
We use capillary electrophoresis, HPLC, UV spectroscopy, and mass spectrometry to confirm sequence accuracy, integrity, capping efficiency, and poly(A) tail characteristics.
We perform in vitro translation assays and reporter gene systems to evaluate protein expression levels and confirm the mRNA performs as designed in biological systems.
We implement nucleotide modifications, optimized cap structures, and sequence engineering including codon and UTR optimization to reduce degradation and extend functional longevity.
Tailored mRNA sequences enable precise protein expression, improve experimental reproducibility, and integrate with advanced delivery platforms for diverse research needs.
References