The stability and functional performance of mRNA molecules depends on essential nucleotide modifications. The presence of these nucleotide alterations such as m6A, pseudouridine (Ψ), m5C, ac4C, Nm, and internal m7G has substantial effects on mRNA metabolism. The most common internal mRNA modification known as m6A undergoes dynamic regulation by specific enzymes which then affect mRNA stability as well as splicing and translation and export processes. Pseudouridine shields mRNA from breakdown thereby increasing its stability and modifies its translation process. The chemical alteration m5C which NSUN2 enzymes catalyze helps stabilize mRNA through the recruitment of particular RNA-binding proteins. The new modification ac4C strengthens mRNA stability and translation performance with the most significant effects observed near the translation initiation codon. Nm remains a protective element against exonucleases particularly in the first two transcribed nucleotides of mRNA. RNA secondary structure and ribosome binding experience changes due to internal m7G modifications. Together these modifications serve essential functions in mRNA regulation which influence both gene expression levels and cellular activity. Research advancing basic science and therapeutic practices depends on understanding how these mechanisms and impacts function.
The stability and translational efficiency of mRNA molecules depend heavily on nucleotide modifications which also influence their overall functionality. These modifications affect mRNA metabolism deeply which makes them essential for the optimization of mRNA-based therapeutic approaches.
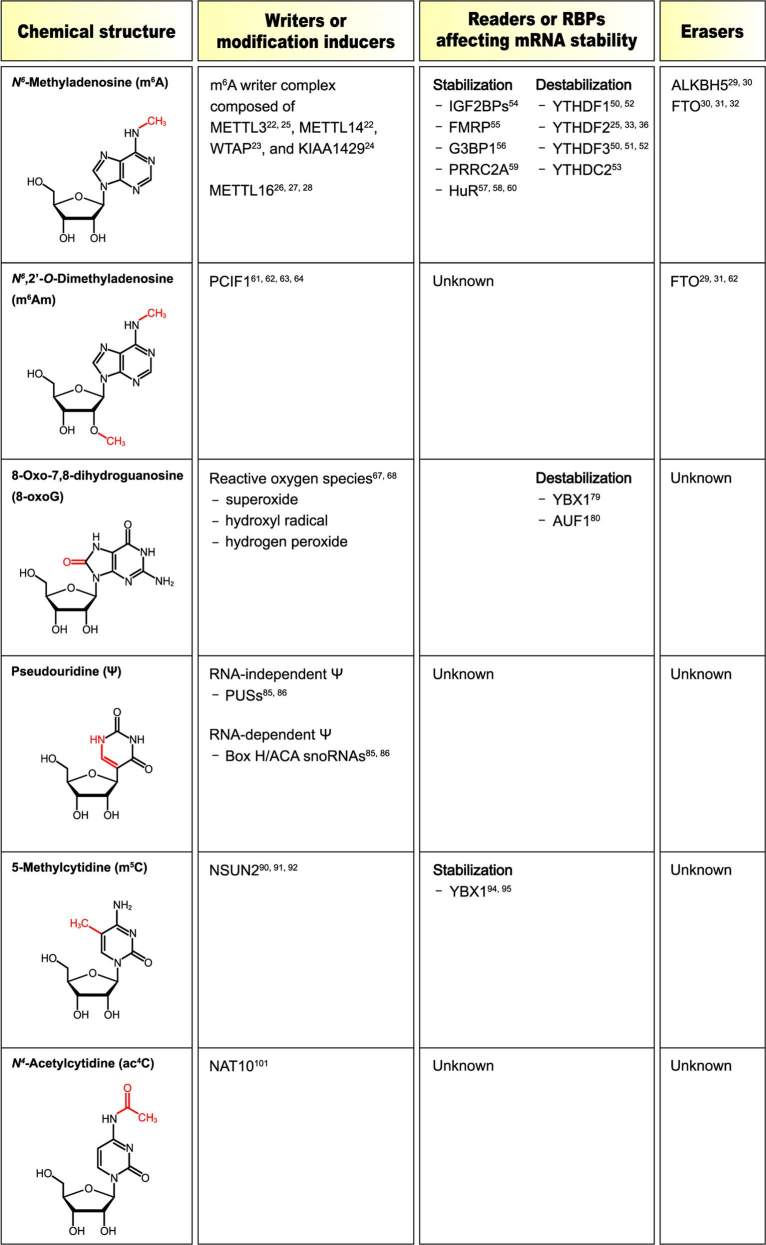 Fig. 1 Chemical structures of RNA modifications affecting mRNA stability.1
Fig. 1 Chemical structures of RNA modifications affecting mRNA stability.1
Through base-specific isomerization reactions driven by pseudouridine synthases (PUSs) activity Ψ emerges as a derivative of uridine (U). There are 13 PUSs found in humans. Ψ appears in ncRNA and mRNA molecules and maintains its presence across different species. Two distinct molecular pathways drive the formation of Ψ. Single protein enzymes known as PUSs serve as the first mechanism which recognize their substrate and perform the isomerization of uridine into Ψ through RNA-independent pseudouridylation. A second mechanism which relies on RNA and four core proteins operates differently from the single protein enzyme mechanism. The RNA component serves as a guide pairing with the substrate RNA to direct core protein enzyme Cbf5 in performing the pseudouridylation reaction at a designated site. The transformation from uridine to pseudouridine remains permanent because it cannot be reversed. Pseudouridine Ψ possesses an additional hydrogen-bond donor compared to uridine at its non-Watson-Crick edge. Its incorporation into RNA modifies the chemical and physical properties which leads to alterations in cellular function.
m1Ψ functions as a modified nucleotide that both decreases immune activation and boosts mRNA stability along with translational efficiency. This modification offers significant advantages for mRNA vaccines because it prevents inflammatory reactions and enhances protein production.
RNA molecules can undergo post-transcriptional modifications at their cytosine bases. Cytosine molecules transform into 5-methylcytidine (m5C) and 3-methylcytosine (m3C) through specific modification processes. Through C-to-U RNA editing cytosine base pairs in RNA molecules are converted into uridine. RNA m5C methyltransferases (RCMTs) produce the m5C RNA modification through the action of proteins from the NOL1/NOP2/SUN domain (NSUN) family and the DNA methyltransferase homologue DNMT2. Scientists recognize the m5C RNA modification as a reversible biochemical process. Enzymes known as ten-eleven translocation (TET) family proteins catalyze the removal of m5C by oxidizing it into cytosine-5-hydroxymethylation (hm5C) within RNA. YBX1 and ALYREF function as m5C reader proteins.
The m6A modification represents the most common internal mRNA change and influences multiple cellular and physiological pathways including maternal-to-zygotic transition (MZT) and cortical neurogenesis while also regulating cancer stem cells in acute myeloid leukemia. Through transcriptome-wide analysis researchers discovered that m6A sites (Gm6AC or Am6AC) occur in noncoding RNAs and mRNAs with higher concentrations appearing in long exons and near stop codons. The methyltransferase complex made up of methyltransferase like 3 (METTL3), METTL14, WTAP and KIAA1429 modifies nascent transcripts with m6A during transcription. The methyltransferase enzyme complex performs methylation by attaching a methyl group to the N-6 position of the adenosine base. METTL16 represents the second m6A writer protein which participates in m6A modification for both coding and noncoding RNAs. METTL16 modifies only a few mRNAs including MAT2A mRNA among its known substrates. The m6A erasers (demethylases) function to remove the methyl group from m6A which results in the reversible transformation into adenosine. ALKBH5 functions as the main and exclusive m6A demethylase within the α-ketoglutarate-dependent dioxygenase family. FTO demethylase demonstrates a limited preference toward m6A modification.
RNA 2′-O methylation (Nm modification where N stands for any nucleotide) serves as a standard RNA modification present across different RNA types including rRNA, tRNA, mRNA and small non-coding RNAs such as miRNAs and siRNAs. The ribose of nucleotides receives an Nm modification by having a methyl group covalently connected to its 2′ hydroxyl group during either co-transcriptional stages or post-transcriptional steps. The production of this RNA modification occurs through either autonomous methyltransferases or through the enzyme fibrillarin which operates under snoRNA guidance. The Nm RNA modification is believed to introduce structural changes that produce more stable RNA molecules and affect RNA cellular functions. Research revealed that inflammatory processes drive snoRNA to move outside the nucleus and RNA-Seq data showed snoRNAs to be present in cells' extracellular vesicles. The available information supports the notion that snoRNAs function beyond their current known roles to facilitate cell-cell communication. The 2′-O methylation at nucleotide 1 characterizes most mammalian mRNAs resulting in cap 1 mRNA.
The RNA modification known as m3C exists in both tRNA and mRNA molecules. Research findings indicate that eukaryotic cells display a widespread presence of the m3C RNA modification at position C32 within tRNAThr and tRNASer molecules. RNA methyltransferases form a diverse enzyme family that delivers a methyl group from SAM to multiple RNA sites. Mammals have four METTL enzymes which include METTL2A, METTL2B, METTL6 and METTL8. METTL8 functions as the enzyme responsible for m3C modification in human mitochondrial tRNAs. Two distinct methyltransferases Trm140 and Trm141 exist in fission yeast yet budding yeast hosts only the single methyltransferase Trm140. The m3C RNA modification is reversible. Human cells contain two demethylase enzymes known as ALKBH1 and ALKBH3. ALKBH1 acts as a demethylase for human mRNA by removing methyl (CH3) groups while ALKBH3 performs the same function for human tRNA. The ALKBH3 enzyme functions as a m1A demethylase for human tRNA.
Molecular stability of mRNA increases substantially with nucleotide modifications as these alterations protect against nuclease degradation. The improved stability of mRNA molecules serves as a crucial factor to maintain their structure and enhance their functionality for medical applications. The modifications Ψ and m5C increase RNA-RNA duplex thermal stability which results in mRNA protection against degradation. The modifications described above not only protect mRNA but also reinforce its backbone to enhance stability. The improved stability of mRNA allows it to remain functional inside cells which results in more efficient protein production.
mRNA therapeutic applications encounter substantial difficulty because they have the capacity to trigger immune system defenses. Altering mRNA nucleotides reduces its immunogenic properties which lowers its ability to trigger the innate immune response. The use of nucleotide modifications Ψ and m1Ψ suppresses immune receptor activation of TLR7/8, PKR, and RIG-I thereby reducing the likelihood of negative immune responses. These mRNA modifications enable the molecule to bypass immune detection thereby preventing degradation and avoiding unwanted immune responses. The ability to reduce immune system activation is crucial for ensuring the safety and effectiveness of mRNA vaccines.
Modifying nucleotides in mRNA can improve translation efficiency resulting in increased protein production levels. The application of modifications like Ψ and m1Ψ enhances translation efficiency through better ribosome attachment and decreased ribosome stalling. Research studies show that mRNA modified with Ψ leads to significantly higher protein expression levels than unmodified mRNA. The m1Ψ modification increases translation efficiency through a process dependent on eIF2α. When these modifications are applied they boost mRNA longevity while improving protein translation which proves critical for therapeutic usage.
At BOC Sciences, we specialize in custom synthesis and formulation of mRNA using a wide range of chemically modified nucleotides. Our goal is to support your mRNA therapeutic development with enhanced expression, stability, and reduced immunogenicity-tailored precisely to your application's needs.
We offer high-quality, research- and GMP-grade modified nucleotides for in vitro transcription (IVT), including but not limited to:
All nucleotide chemistries are verified by rigorous analytical methods to ensure purity, incorporation efficiency, and batch consistency.
We provide fully customized IVT mRNA synthesis services incorporating one or more modified nucleotides:
Our synthesis solutions are guided by your research goals:
All mRNA products undergo:
Whether you're exploring novel modifications or need GMP-ready synthesis of a validated therapeutic, our flexible platforms and scientific expertise ensure your modified mRNA performs to its fullest potential.
Partner with us for end-to-end synthesis solutions that accelerate your mRNA innovation.
Incorporation of Nm enhances ribosome attachment and reduces stalling, leading to substantially higher translation efficiency and elevated protein expression levels.
Modifications effectively suppress innate immune receptor activation, allowing mRNA to avoid immune detection while maintaining functional expression.
We provide comprehensive services including sequence design consultation, optimized molar ratios of modified nucleotides, cap analog optimization, and scalable IVT synthesis with rigorous quality control.
We use HPLC, LC-MS, and electrophoresis to confirm nucleotide incorporation, sequence integrity, and batch-to-batch consistency, ensuring reliable mRNA performance.
We customize modification patterns to achieve rapid expression, prolonged half-life, or controlled translation kinetics for vaccines, protein production, or gene editing applications.

References