On November 21, 2019, the US FDA announced that it has approved the listing of Alnylam's RNAi therapy Givlaari (givosiran) for the treatment of acute hepatic porphyria (AHP). This is the second new RNAi drug on the market after Onpattro (patisiran).
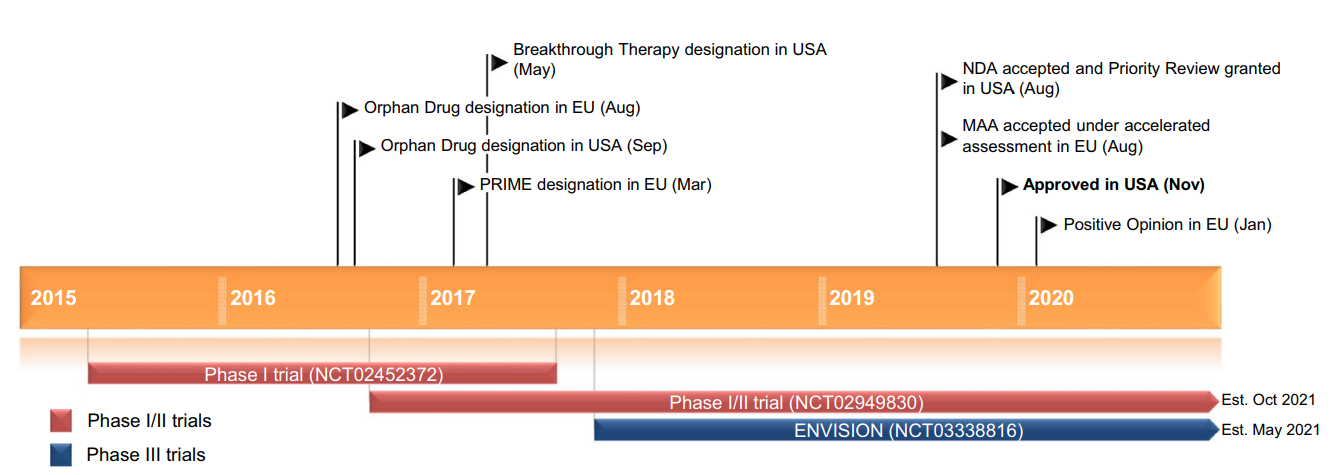 Figure 1: Key milestones in the development of givosiran for the treatment of acute hepatic porphyria (Scott L.J. 20020).
Figure 1: Key milestones in the development of givosiran for the treatment of acute hepatic porphyria (Scott L.J. 20020).
AHP is a rare genetic disease. Due to the absence of specific enzymes in the heme biosynthesis pathway in the liver, patients accumulated neurotoxic heme intermediates aminolevulinic acid (ALA) and bilirubin (PBG). This accumulation can cause acute attacks, called porphyria attacks, which can cause severe pain and paralysis, vomiting, respiratory failure, seizures, and other neurological diseases. These attacks occur suddenly and may cause permanent nerve damage and death. In addition, long-term complications and comorbidities of AHP may include hypertension, chronic kidney disease, or liver disease including hepatocellular carcinoma.
About one-fifth of people worldwide are affected by porphyria. But before this, there is no effective treatment for the disease.
Givlaari is a small interfering RNA (siRNA) guided by aminolevulinic acid synthase 1 (ALAS1), which enhances its efficacy and durability through enhanced stable chemical ESC-GalNAc conjugation technology, and can specifically deliver siRNA to the liver cell. Givlaari destroys ALAS1 mRNA in liver cells through RNA interference (a cellular process that silences genes) and reduces the accumulation of neurotoxic δ-aminolevulinic acid (ALA) and porphobilinogen (PBG) associated with acute porphyria.
Givlaari's approval is based on the results of clinical trials in 94 patients with acute hepatic porphyria. Compared with patients receiving placebo, patients receiving Givlaari had a 70% reduction in the incidence of porphyria, as well as a reduction of (ALA) and PBG.
"We believe that the approval of Givlaari represents a landmark event in the development of precision genetic medicine, which provides new hope treatments for patients with their AHP debilitating manifestations and unpredictability of AHP episodes and their caregivers and doctors who diagnose and diagnose AHP These patients, "said Dr. John Maraganore, CEO of Alnylam.
Now, the field of RNAi new drug development has finally ushered in its own spring. We expect this innovative mechanism to bring us more treatments and benefit most patients.
GalNAc conjugation enhances siRNA uptake by liver cells through receptor-mediated endocytosis, increasing target specificity and experimental reproducibility in hepatocyte-focused research.
Challenges include optimizing chemical modifications for stability, minimizing off-target effects, and selecting sequences that efficiently silence the intended gene in hepatocytes.
Chemical modifications, such as 2'-O-methyl or phosphorothioate linkages, protect siRNA from degradation, enhance binding affinity, and improve consistency in experimental outcomes.
Key factors include delivery efficiency, cellular uptake, target gene accessibility, and compatibility with in vitro or in vivo liver models for robust experimental results.
Custom synthesis and design reduce development time, provide sequence optimization for specific liver targets, and enable rapid validation of gene function or pathway modulation in hepatocyte systems.


Ref: