Gene therapy has emerged as a transformative medical technology with the potential to correct genetic defects, modulate gene expression, or introduce entirely new functions into a patient's cells. Among the most widely used delivery systems for gene therapy are adeno-associated virus (AAV) and lentiviral vectors, both of which have been successfully applied in numerous clinical trials and commercial therapies. These viral vectors are engineered to carry therapeutic genes into target cells, enabling long-term expression and therapeutic benefit.
However, designing effective viral vectors involves far more than inserting a gene of interest into a plasmid. Every element of the vector—from the promoter to the payload size, to the arrangement of enhancer sequences—must be carefully designed for safety, efficiency, and manufacturability. Synthetic DNA technology now plays a central role in this process, enabling researchers to create precisely tailored vector constructs that overcome the limitations of natural sequences and traditional cloning methods.
This article explores the importance of thoughtful vector design in gene therapy, how synthetic DNA enhances viral vector engineering, and the services best suited to support researchers developing vectors.
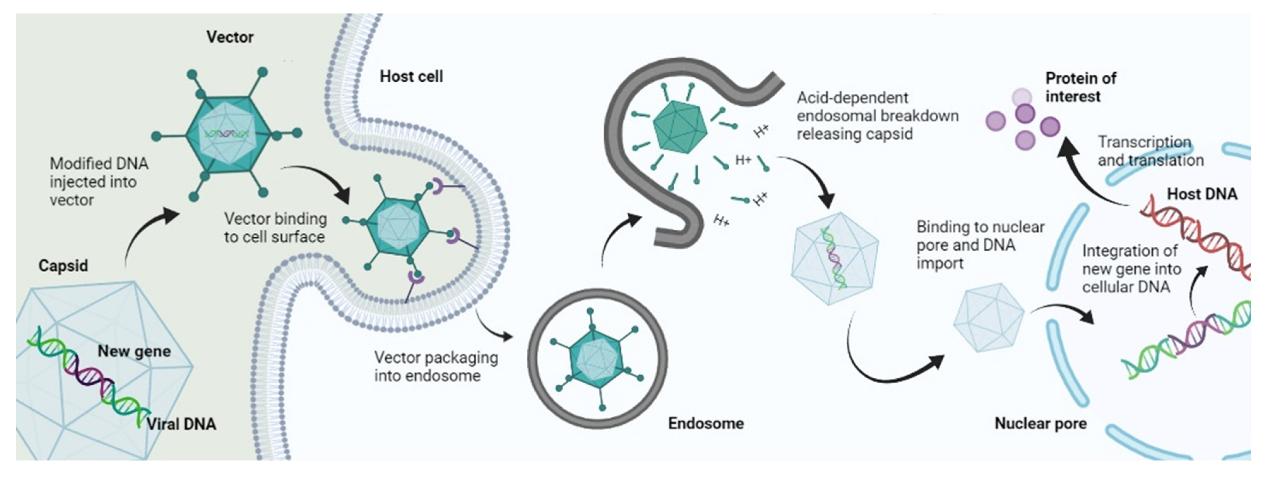 Fig.1 The delivery process for gene on viral vectors1,2.
Fig.1 The delivery process for gene on viral vectors1,2.
Gene therapy is transforming the landscape of modern medicine by enabling precise correction of genetic defects at their source. Central to this approach is the use of vectors—engineered delivery systems that transport therapeutic genes into target cells. However, a vector is far more than just a carrier; its structure directly influences the therapy's safety, efficiency, and durability. A poorly designed vector can limit the amount of genetic material delivered, destabilize during production, or fail to drive appropriate gene expression once inside the body. Therefore, careful attention to vector design is critical for the success of any gene therapy program. Key design considerations include managing payload capacity constraints, ensuring sequence stability, and optimizing regulatory elements such as promoters and enhancers. Each of these factors must be balanced to create vectors that are both functional and clinically viable. These factors are described in detail in the following section.
One of the foremost challenges in vector design is the limited payload capacity of commonly used viral vectors. Each vector type has a strict upper limit on the size of genetic material it can carry. For instance, adeno-associated viruses (AAVs)—widely favored for their safety and low immunogenicity—can typically accommodate only ~4.7 kilobases (kb) of DNA. This poses a significant challenge when working with large genes, complex expression cassettes, or therapeutic payloads requiring multiple components (e.g., regulatory elements, reporters, or gene-editing systems like CRISPR/Cas9).
When payloads exceed a vector's capacity, several strategies may be considered, including dual-vector systems (splitting the gene across two vectors), mini-gene constructs (using truncated but functional gene versions), or selecting an alternative vector type with higher capacity, such as lentiviruses (~8–10 kb) or adenoviruses (~30–40 kb). However, each alternative introduces trade-offs in terms of integration risk, immunogenicity, and tissue targeting.
This makes early consideration of payload size and overall construct architecture crucial during vector design. Strategic gene optimization—such as codon usage refinement, compact regulatory elements, and intron removal—can help fit larger functional payloads within limited space without compromising expression efficiency.
Genomic stability is another critical consideration in vector design. Direct or inverted repeats, palindromic sequences, and high GC content regions can lead to recombination events, secondary structure formation, and vector degradation—either during vector production or after delivery into host cells. These sequence elements may also interfere with transcription and translation, reducing therapeutic efficacy.
For example, long terminal repeats (LTRs) in retroviral and lentiviral vectors, while necessary for integration, can sometimes promote recombination if the inserted gene or regulatory sequences contain homologous regions. Similarly, inverted terminal repeats (ITRs) in AAVs are essential for replication and packaging but are susceptible to forming stable hairpin loops, complicating plasmid stability during cloning and large-scale manufacturing.
To mitigate these risks, synthetic DNA technologies can be used to recode problematic regions without altering the encoded protein. These tools allow for removal of unwanted sequence motifs, reduction in repeat elements, and reshuffling of synonymous codons to enhance both stability and expression. Additionally, careful design of the vector backbone—avoiding duplication of promoter or polyadenylation signals—helps maintain plasmid integrity throughout vector production.
Ultimately, robust vector design must anticipate and minimize sequence-based instabilities that could impact manufacturability, delivery, or therapeutic consistency.
The control elements within a gene therapy vector—namely promoters and enhancers—dictate how, when, and where the therapeutic gene is expressed. Optimizing these regulatory sequences is critical to achieving effective treatment outcomes while minimizing off-target effects or immune activation.
A promoter must be carefully chosen based on the therapeutic context. Ubiquitous promoters (e.g., CMV, EF1α, or CAG) drive high levels of expression across a broad range of cell types, making them suitable for systemic diseases or ex vivo-modified cell therapies like CAR-T. However, they can also induce unintended expression in non-target tissues and may be silenced over time in vivo. In contrast, tissue-specific or inducible promoters enable refined spatial or temporal control, reducing the risk of toxicity or immune response. For example, using a neuron-specific promoter in a CNS-targeted AAV therapy can enhance safety by restricting expression to relevant cells.
Enhancers further modulate transcriptional activity by boosting promoter output, often in a cell-type-specific manner. Synthetic or engineered enhancers, derived from combinatorial screening or genomics data, can provide significant advantages over naturally occurring elements in terms of size, strength, and specificity.
In optimizing promoters and enhancers, balancing expression strength with precision is key. Overexpression can lead to cellular stress or immune detection, while underexpression may render the therapy ineffective. Additionally, vector capacity constraints often force trade-offs between regulatory complexity and payload size—making concise, high-efficiency elements desirable.
Codon optimization and mRNA stability elements (e.g., Kozak sequences, UTRs) may also be incorporated into the expression cassette to further fine-tune translation and protein yield.
The development of viral vectors for gene therapy demands precise genetic control, rapid iteration, and robust production of often complex DNA constructs. Traditional cloning methods, while reliable, can be time-consuming, limited by sequence constraints, and inefficient for high-throughput variant generation. DNA synthesis technologies have revolutionized viral vector engineering by enabling the rapid, accurate, and customizable production of genetic elements tailored to specific therapeutic goals. Whether optimizing gene expression, modifying vector backbones, or testing new variants, synthetic DNA allows researchers to overcome long-standing barriers in molecular design and accelerate the path from concept to clinic.
One of the key advantages of synthetic DNA is the ability to codon-optimize genes for specific host cell types. Codon usage varies between species and even between tissues; by redesigning a gene's coding sequence to align with the codon preferences of the target cell, researchers can significantly improve protein expression levels without altering the amino acid sequence.
For viral vectors, this optimization is especially important. A gene therapy product intended for muscle, liver, or neuronal tissue may require entirely different codon usage profiles to achieve efficient translation. DNA synthesis allows developers to apply bioinformatics-driven codon optimization algorithms that account not only for frequency but also for GC content, mRNA secondary structure, and rare codon avoidance. The result is enhanced protein yield, improved vector potency, and reduced variability in expression—factors that are critical for both preclinical efficacy and clinical scalability.
Furthermore, synthetic codon optimization can also reduce immunogenicity by eliminating cryptic splice sites or immune-activating motifs, thereby improving safety profiles in therapeutic applications.
Many sequences required in modern gene therapy vectors—such as highly repetitive elements, strong secondary structures, or high/low GC content regions—are notoriously difficult to clone using conventional restriction enzyme and PCR-based methods. These problematic regions can lead to instability in bacterial hosts, low yields during plasmid amplification, or unintended rearrangements.
DNA synthesis circumvents these limitations by allowing the direct chemical assembly of virtually any sequence, regardless of complexity. This makes it possible to incorporate novel genetic elements such as artificial promoters, self-cleaving peptides, polycistronic cassettes, or introns that would otherwise be challenging to clone or maintain in traditional systems.
For example, synthetic DNA enables the construction of dual-expression systems (e.g., transgene + reporter), complex regulatory modules with tissue-specific control, or modified viral capsid genes for targeted delivery. Developers can also iterate on vector designs that include multiple therapeutic payloads, safety switches, or enhancer elements—without the bottlenecks typically associated with molecular cloning.
By eliminating the cloning constraints that often slow vector innovation, DNA synthesis empowers scientists to build sophisticated constructs that were previously out of reach.
In gene therapy development, rapid iteration is essential. Whether optimizing vector tropism, enhancing expression levels, or minimizing immunogenicity, developers often need to test numerous genetic variants in a short period of time. Traditional cloning methods, however, are labor-intensive and time-consuming, slowing down the design-build-test cycle.
DNA synthesis dramatically accelerates this process by enabling on-demand generation of diverse vector variants, all precisely tailored to experimental goals. Researchers can quickly produce libraries of constructs with controlled variations—such as different promoters, regulatory elements, codon-optimized transgenes, or capsid mutations—without the delays and limitations of manual cloning. This agility is particularly valuable when screening for optimal expression cassettes or evaluating payload performance across different cell types and delivery routes.
For example, when engineering AAV vectors for tissue-specific delivery or immune evasion, synthetic DNA allows for the fast and parallel synthesis of capsid variants or expression modules. Similarly, lentiviral systems can be rapidly modified to test combinations of internal promoters, safety elements, or splice enhancers.
The result is a faster feedback loop between design and validation, allowing developers to identify lead candidates more efficiently, adapt to emerging data, and move promising constructs into preclinical or clinical pipelines with minimal delay. In a field where timelines are critical and innovation is key, fast DNA synthesis enables a truly agile approach to viral vector engineering.
The complexity of viral vector engineering—especially in gene therapy and vaccine development—requires robust, high-precision tools to ensure success from early research through clinical production. Whether you're designing AAV, lentiviral, or adenoviral vectors, working with long or complex constructs, or preparing sequences for regulatory submission, our synthesis and cloning services are built to meet the exacting needs of viral vector researchers. Below are three of our most recommended services for accelerating and de-risking your development pipeline.
Our long construct synthesis service enables seamless generation of complex DNA sequences up to 10 kb, ideal for viral vectors carrying multi-gene payloads or regulatory modules. This eliminates cloning bottlenecks, ensures structural integrity, and allows for rapid delivery of ready-to-use plasmids tailored to AAV, lentiviral, or adenoviral systems.
We offer high-fidelity plasmid construction with 100% sequence verification, minimizing the risk of mutations or frame shifts that could compromise viral vector performance. Each plasmid is rigorously QC-checked and delivered ready for packaging, transfection, or further modification—ensuring reliability from bench to clinic.
Our services support regulatory-aligned development by offering secure sequence tracking, full documentation, and optional GMP-like compliance. Ideal for preclinical and IND-stage vector projects, we help ensure your constructs meet quality and traceability standards required for clinical advancement.
A well-designed viral vector ensures stable gene expression, efficient payload delivery, and robust manufacturability. Poorly constructed vectors may compromise expression or structural integrity during production.
Synthetic DNA enables codon optimization, removal of unstable sequence motifs, and assembly of complex constructs, allowing researchers to create precise and customizable vectors without traditional cloning limitations.
Strategies include dual-vector systems, mini-gene constructs, or choosing alternative vectors with higher capacity. Codon optimization and compact regulatory elements also help fit larger genes into constrained spaces.
Choosing tissue-specific or inducible promoters and high-efficiency enhancers balances expression strength with precision, reducing off-target activity and ensuring the gene is expressed in intended cells.
Direct DNA synthesis allows assembly of repetitive, GC-rich, or structurally complex sequences, supporting multi-gene cassettes, regulatory modules, and novel capsid designs that are difficult to generate via conventional cloning.
On-demand synthesis of diverse constructs enables fast iteration, parallel testing of promoters, enhancers, or capsid modifications, and quick identification of optimal vectors, reducing development time for research projects.
References