Two decades of siRNA carrier optimization later, most of the flashy performers in vitro still buckle up when faced with the anatomical, biochemical and regulatory complexity of intact organisms. Attrition is not stochastic; the loss of potency follows a reproducible failure cascade that starts in the petri dish and funnels through scale-up suites, animal facilities and, ultimately, clinical wards. Early discovery is based on transformed cell lines with hyper-permeable membranes, serum-free media that protect naked RNA from nucleases and reporter assays that turn sub-therapeutic knock-down into a photonastic signal. This string of permissive bottlenecks masks the fragility of many formulations such that the same particles, once exposed to whole blood, interstitial pressure or macrophage surveillance, become devoid of potency. Identifying this predictable attrition as a systems problem rather than a single technical bug has re-oriented the field toward an iterative validation pipeline that integrates physicochemical stress tests, immune-competent animals and manufacturability gates before a sequence is even declared lead-worthy. The two sections below focus on the two most lethal translational chokepoints, namely model-induced false positives and scale invariant production pitfalls, and highlight emerging solutions that bring lab elegance closer to clinical pragmatism.
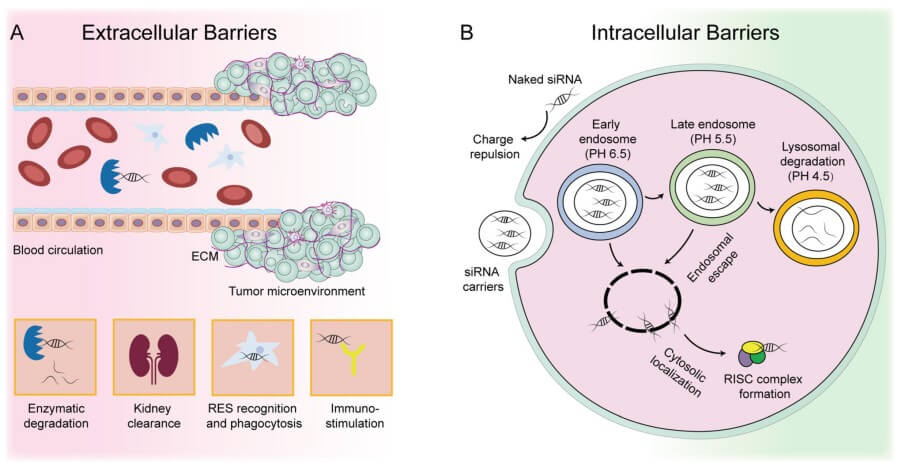 Fig. 1 Extracellular and intracellular barriers of siRNA-based cancer therapeutics.1,5
Fig. 1 Extracellular and intracellular barriers of siRNA-based cancer therapeutics.1,5
Translation failure is often due to a conflict between reductionist paradigms that nurture siRNA carriers and the complex, multi-barrier environments of the human body. Academic deliverables focus on next-iteration improvements to knock-down efficiency, as assayed in simplified conditions. But payers and regulators have come to expect months of consistent silencing in heterogeneous populations, with no off-target effects. Jumping this gulf is a perilous process, because each additional level of biological complexity (protein adsorption, endothelial adhesion, organ-specific clearance, innate immune activation, etc.) tends to filter out sub-optimal designs, exponentially multiplying the impact of small inefficiencies in formulation. As a result, a nanoparticle that can knock down 90% of target mRNA in a plastic petri dish may at best provide a fleeting 20% silencing effect in vivo, a performance gap that repels investors and has stoked the persistent notion that RNAi is not druggable outside of the liver. Separating hype from inherent limitation involves parsing where (and why) the delivery cascade is most likely to break down.
Cell-culture plates are a weak model for 3D tissues. Since in vitro monolayers are void of the tortuosity of the extracellular matrix, which physically limits particle diffusion, carriers there seem to be able to penetrate when their diameter is above the physiological cutoff value for pores. In a tumor xenograft, the same constructs would get stuck in the perivascular rim and the result is a large concentration gradient with hypoxic clones left untouched and a read-out of efficacy that is biased to the peripherally located cells. Immune privilege is another artefact. The majority of screening is done in athymic rodents whose macrophage populations are compromised, so they miss the very rapid opsonization and splenic sequestration that would be found in an immune-competent host. The lack of complement proteins in serum-free media also means that cationic formulations are spared the precipitin reactions that can lead to anaphylactoid responses in primates. Endocytic uptake is also mis-modelled; many transformed cell lines over-express both clathrin and caveolin leading to a large exaggeration of internalization rates and subsequent over-confidence in formulations that target receptors that are in fact scarce in primary tissue. Scaling the dose introduces another distortion. Bench-top transfection reagents are used at micromolar concentrations, many orders of magnitude above what circulation can tolerate, so researchers unwittingly select particles whose potency cannot be decoupled from cytotoxic membrane perturbation. Iterative models now embed multicellular spheroids into microfluidic channels to recapitulate interstitial flow, incorporate human whole-blood loops for real-time complement activation and utilize humanized mice whose macrophages have authentic scavenger receptors. These orthogonal stress tests disqualify formulations whose success relies on the idiosyncrasies of plasticware so only architectures robust across immunological, biomechanical and metabolic variability move toward scale-up suites.
Synthesis at the lab-scale is artisanal: lipid films are manually swirled, microfluidic chips are washed with solvents never challenged for environmental impact, and output is in milliliters, the volumes of a fume hood. The clinic requires liters per hour, upscaling that subjects one to heat-transfer boundaries, lipid peroxidation and particulate contamination that are not part of academia's standard expectations. Ionizable amines, the molecular groups that confer endosomal escape, are easily peroxidized on stainless-steel surfaces at higher temperature, giving rise to aldehydic side products that stimulate toll-like receptor-4 and derail the immune-neutral credential earned in mice. The same can be said of microfluidic channel fouling: lipid formulations that nicely self-assemble in glass capillaries will gel on nickel-phosphor coated surfaces to give polydispersity indices out of pharmacopoeia bounds and clog filters designed to be sterile barriers. This extends even to the solvents of choice: chloroform is quick to use for proof-of-concept experiments but subjected to low residual limits in industrial settings that require, among other things, expensive cascades of vacuum stripping. Regulators now expect control strategies for every critical quality attribute – encapsulation efficiency, zeta potential, residual solvent, endotoxin – in a design space that has already been reduced by cycles of risk assessment. To that end, solutions are being developed in the form of continuous-flow tangential micro-mixers with limited thermal history, single-use polymer flow paths that eliminate metal contact, and in-line Raman probes capable of real-time monitoring of lipid phase behavior. Building in such engineering countermeasures early on can avert the frustrating case in which a candidate that shines in fluorescent mice fails to deliver on its own reproducibility, leading to translational failure by biology and not by a test-tube alchemy.
The beauty of the RNAi machinery has never been in question, but the path from a sequence with a good in vitro profile to a commercial product has many attrition steps that tend to have common causes. Moreover, these challenges are not just scientific or manufacturing in nature, but are also in regulatory science, and the overlap of these aspects can multiply the effect of individual challenges. For example, a potent and selective formulation reducing a target transcript in a publication may fail to reproduce in another lab due to variation in endotoxin, lipid oxidation or serum protein profile altering the nanoparticle corona and affecting biodistribution. If reproducibility is demonstrated, the regulatory evidence package required can extend beyond the small-molecule development framework in place for decades, requiring developers to create new toxicology paradigms for these biologic–chemical combination products. Finally, transition from a gram scale academic synthetic route to a kilogram scale GMP process exposes all weak chemical linkages to higher temperatures, metal surfaces and residual solvents that may have been innocuous at the microscale. The following sections will highlight some of these potential choke points.
 Fig. 2 Manufacturing challenges of siRNA therapeutic agents.2,5
Fig. 2 Manufacturing challenges of siRNA therapeutic agents.2,5
Failures of reproducibility are seldom the result of a single smoking gun variable. The problems come from a gradual accumulation of small, semi-obscured parameters, each of which is individually innocent, but together, deadly. Lipid mixes made from different batches of chloroform solvent can contain picogram levels of peroxide, enough to oxidize an ionizable amine, shifting the pKa of the whole nanoparticle by a few tenths of a unit. The resulting shift in surface charge might not influence particle size or encapsulation efficiency (outputs which are often reported) but it reroutes particle clearance from renal to hepatic macrophages, obliterating therapeutic exposure, without a single warning metric flickering red. In another case, the source of serum that was used during an in vitro potency assay influenced corona composition; foetal bovine serum from one supplier may have had higher titers of complement C3. This triggers opsonization which is misinterpreted as cytotoxicity, and the scientists throw away a perfectly good formulation. Even the shape of microfluidic mixers can be a source of variation; a chip that works perfectly in a 25 °C room could produce polydisperse vesicles after ambient temperature increases a few degrees, because lipid solubility is steeply temperature-dependent. Next-generation protocols have attempted to address this drift by pre-emptively embedding quality-by-design checkpoints. Lipid stocks are qualified by peroxide value, rather than just expiration date; in-house serum banks are created by pooling and pre-screening for opsonin levels; and microfluidic rigs are equipped with inline temperature probes whose readings are logged for every batch. Raw data-sets (not just the averaged endpoints) are being deposited in public repositories too, which allows cross-lab meta-analyses that can statistically associate subtle procedural differences to outcome failures. By approaching reproducibility as a multivariate optimisation problem, rather than a moral imperative, the community is slowly transforming artisanal bench craft into an engineering discipline with a variance narrow enough to survive external audit.
The siRNAs are considered both chemical and biological agents. Therefore, they face regulatory requirements for both and their respective guidelines that may have been written long before their advent. This issue extends to both physicochemical and biological issues, and frequently sponsors need to satisfy guidelines both for small molecules as well as oligonucleotides. For example, an agency might ask for an impurity profile at 0.1% level, which is typical of small molecules, while asking for transcriptome-wide off target effects, which is more typical of gene-therapy products. Another example is the requirement for chronic toxicology. SiRNA duplexes are stable for weeks in renal cortex, yet, their metabolites cannot be distinguished from endogenous nucleotides. Therefore, it is not clear whether parent drug or metabolite-driven exposures should be used. Immunogenicity is another issue. Trace endotoxin or dsRNA contaminants in reagents can lead to interferon responses which can be confused with on-target pharmacology, which can result in apparently high no-observed-adverse-effect levels which fall apart in larger more ethnically diverse populations. Finally, regional differences make global development more challenging. For example, one region might be willing to accept three-month rodent data for accelerated approval, while another region requires six-month canine studies. The resultant parallel programs can cost more than the chemical optimization effort itself. This and other problems can often be addressed through iterative scientific advice with a presentation of underlying mechanisms (for example, chemical modification maps that prevent toll-like receptor activation) in order to help shape toxicology packages to agency expectations before the studies are done.
GMP compliance mercilessly subjects every delicate chemical subtlety of siRNA to the brutal thermodynamics of bulk process reactors. A formulation that self-assembles spontaneously into monodisperse nanoparticles when casually hand-swirled in a glass flask can precipitate into gels when agitated at pilot-scale impeller speeds, since shear rates that are inconsequential at milliliter scales become disastrous at liter scale, tearing lipid bilayers irreversibly apart. Metal-ion contamination – benign in research-grade solvents – promotes oxidative cleavage of PS linkages, producing aldehydic byproducts that activate macrophages and negate months of pre-clinical safety work. Even scaling up from nitrogen-blanketed vials to stainless-steel tanks can introduce headspace oxygen levels that tip the ratio of ionizable to protonated lipid species, changing particle zeta potential in ways that cannot be fixed by sterile filtration. Cleaning validation, rarely considered in academic synthesis but key for batch manufacturing, can become a horror show if siRNA sticks to tank walls through electrostatic interactions that cannot be removed by conventional caustic cleaning cycles; residual oligonucleotide can then redissolve into the next batch, seeding cross-contamination that can only be detected by qPCR. Container–closure integrity is another wrinkle; the same rubber stoppers that reliably maintain antibody stability can leach sulfur accelerants that can react with unprotected ribose to form adducts whose immunogenicity is unknown. Emerging mitigation approaches include single-use polymer flow paths that eliminate metal contact, continuous tangential-flow micro-mixers that dramatically reduce residence time distributions, and in-line spectroscopic probes that can detect lipid phase behavior in real time, flagging deviations as they occur so that they can be corrected before they become entrenched. By hardwiring such engineering controls in from the beginning, developers can avoid the disheartening situation in which a candidate that is being developed as a next-generation breakthrough collapses under the weight of its own manufacturability, and instead ensure that GMP compliance becomes a validation of elegance rather than the executioner.
Despite a dizzying array of chemical modifications and carrier chemistries now catalogued in the siRNA field, the majority of these candidates die during the journey from curated academic environments to the stochastic realities of scale-up, regulation and human dosing. The few which survive share a common design philosophy: they approach translational risk as an independent variable to be engineered against, rather than a fate to be accepted. This mindset has given rise to a new generation of "smart" strategies that embed quality-by-design into every hierarchical layer—from the angstrom-scale positioning of a 2′-methyl group to the meter-long stainless-steel fluidic path. By synchronizing nanoparticle optimization with scalable manufacturing protocols and by generating pre-clinical data packages that anticipate regulatory objections before it is raised, developers are converting the attrition funnel into a controlled manufacturing line in which elegance and robustness are no longer mutually exclusive.
Modern optimization moves away from the historic approach of maximal knock-down in a single cell line, and instead targets a multi-dimensional sweet spot defined by potency, stability, immunological inertness and manufacturability. One component is compositional asymmetry: instead of packing the particle with cationic lipid, the ionizable amine is titrated down to the lowest mole fraction that still enables complete encapsulation, thereby minimizing complement activation while maintaining pH-triggered endosomal escape. A second is morphological anisotropy; rod-shaped or polyhedral geometries whose long axes are nominally aligned with collagen fibers can penetrate more deeply into tumour tissue than spheres of identical hydrodynamic diameter, but can still be sterile-filtered if the minor axis is maintained below the pore cut-off. Surface architecture is a third: zwitterionic phosphorylcholine polymers are grafted at sub-brush density so that stealth behavior is maintained without inhibiting ligand exposure after the corona sheds in the acidic interstitium. Critically, these adjustments are not performed in a sequential or siloed manner, but rather through Design-of-Experiment matrices in which lipid identity, ligand density and PEG length are varied simultaneously, such that non-linear interactions (e.g. that modest PEGylation can actually enhance ligand binding by reducing non-specific protein masking) can be captured and modelled. The resulting particles are retained across species, are resistant to serum-induced aggregation for days, and can be lyophilized without cryoprotectants, thereby collapsing cold-chain requirements and enabling decentralized deployment. Perhaps most importantly, the optimized composition is then locked into a master formula whose critical quality attributes have been defined a priori, ensuring that every subsequent scale or site change is a validation exercise rather than a new discovery campaign.
Conventional flask-based methods can yield beautiful nanoparticles reproducibly at the milliliter scale, but shear profiles and thermal homogeneity change in an uncontrolled fashion when scaled up to stirred-tank reactors. Continuous microfluidic mixing is now the preferred solution to this problem, but initial devices suffered from low throughput and clogging when used with organic solvents. Current systems bypass these issues by mass-producing chips that contain staggered herringbone mixers in parallel behind precision flow resistors; the resistors balance pressure across several dozen micro-channels, so each mixer experiences the same flow rates and the entire array can be scaled to liters per hour output without further optimization. Particle size and polydispersity do not change across scales as mixing time is determined by channel dimensions rather than by total volume, avoiding the expensive re-validation work that limits batch operations. Stainless-steel flow paths are swapped for single-use polymer cartridges to meet Good Manufacturing Practice requirements, eliminating carry-over risk and minimizing cleaning validation to a leak test. In-line absorbance and Raman probes measure lipid phase transition and encapsulation efficiency in real time, allowing outlier batches to be rejected on the fly rather than quarantined after the fact. Waste reduction measures can be taken to assuage environmental concerns about solvent use, including closed-loop ethanol recovery systems that distil organic phase in-house and reduce waste streams by an order of magnitude. The upshot of these changes is a production line whose output can be ramped from discovery (mL/h) to clinical supply (L/h) with little requalification effort by adding more mixer modules to a pre-qualified skid, and so turning the challenge of scale-up into a plug-and-play task.
Poorly aligned preclinical development is frequently identified a posteriori, when regulatory agencies ask for studies to be repeated under marginally different conditions; the repetition can take up enormous amounts of time and money. Forward-thinking development inverts this process by incorporating the agency's thought-process in the planning of the initial experiments. Off-target analyses, rather than a post-hoc attempt at box-checking, are performed transcriptome-wide and across species early on, using doses at least a log higher and lower than what is anticipated to be used in the clinic. Human whole-blood assays are used to interrogate immune safety, rather than murine splenocytes, because complement activation or cytokine release by primates can't be predicted from rodent cell reactivity. Biodistribution is established using both radiolabel and qPCR to ensure concordance, pre-empting the frequent argument that lipid labels don't report on RNA payload. To meet the expectations of Chemistry, Manufacturing and Controls requirements, formulation changes are frozen after an agreed-upon gateway, with any further changes requiring a formal bridging package, the specifications of which have been agreed-upon a priori with the regulators to prevent interminable optimization cycles that drain both budget and reviewer goodwill. Most importantly, developers now engage in rolling scientific advice by iteratively submitting non-clinical summaries so that issues can be raised while studies can still be redesigned, rather than when the archive is locked, turning regulatory review from a binary judgement call into a continuation of the experimental design process.
At BOC Sciences, we understand that translating siRNA formulations from research to clinical application is one of the biggest challenges in RNA therapeutics. Many promising candidates fail during scale-up or regulatory transition-not because of weak efficacy, but due to unstable formulations, process variability, and poor reproducibility. Our mission is to help biopharma innovators bridge this critical gap with scalable, compliant, and high-performance siRNA delivery solutions.
Our delivery experts tackle the fundamental barriers that often delay or derail siRNA programs.
We specialize in overcoming:
Every solution we deliver is backed by rigorous data, ensuring consistency and compliance from bench to batch.
We offer a fully integrated siRNA development pathway designed to reduce risk and time-to-clinic. Our comprehensive services include:
This end-to-end model allows our partners to maintain scientific focus while we manage the technical complexity of delivery development.
Our strength lies in combining innovative formulation technologies with manufacturing excellence. We bring:
With a deep understanding of both scientific innovation and industrial translation, we enable biopharma partners to move confidently from concept to clinic.
At BOC Sciences, we don't just solve today's siRNA delivery challenges - we build platforms for tomorrow's breakthroughs. Our scalable nanoparticle technologies and regulatory-ready processes empower RNA drug developers to accelerate clinical success, minimize development risk, and achieve consistent product quality. Partner with us to transform your siRNA program into a clinically ready therapeutic reality. Contact our experts today to discuss how our scalable delivery solutions can strengthen your next-generation RNA pipeline.
1. Why do many siRNA formulations fail to translate from lab to clinic?
Key reasons include poor scalability, variability in formulation quality, and lack of reproducibility under GMP conditions.
2. What are common bottlenecks in siRNA development?
Challenges include inconsistent particle size, low encapsulation efficiency, stability issues, and complex regulatory pathways.
3. How can these bottlenecks be overcome?
Implementing quality-by-design principles, microfluidic manufacturing, and early regulatory alignment helps ensure scalability and compliance.
References