The path from serendipitous gene knock-down to robust therapeutics is paved with publications: the papers that reported on very potent silencing in vitro usually mention inconclusive toxicities in vivo, often puzzling. Off-target effects are no longer perceived as background noise, but are understood as a design flaw that is amplified by the entire RNAi machinery. High-precision solutions thus consider specificity as a systems parameter: chemical modifications reduce the likelihood of seed-region mismatches; delivery vehicles control biodistribution and restrict the access to target organs; in-silico pipelines predict – and preempt – unwanted transcript interactions long before a single duplex is synthesized. The result is a new generation of siRNA therapeutics whose safety margin is inherent to the molecule's life-cycle, rather than being bolted on late in development.
Off-targets are the cumulative unintended changes in gene expression caused when the guide (rarely passenger) strand hybridizes to transcripts outside of the intended locus. The most prevalent route is miRNA-like: six-to-eight contiguous nucleotides at positions 2–8 pair to complementary sites, largely in 3′ untranslated regions, recruiting Argonaute and TNRC6 proteins to repress translation or hasten deadenylation. Given that such short motifs are repeated so commonly across the transcriptome, a single siRNA can in principle shadow dozens of endogenous microRNAs, casting a ripple of down-regulation that may appear as a specific phenotype. A second, often overlooked route is immunogenic: dsRNA longer than ~23 bp, or sequences enriched in GU-/AU-dinucleotides, activate endosomal TLR-7/8 and cytosolic RIG-I, provoking type-I interferons and a downstream interferon-stimulated gene signature that can overwhelm the primary knock-down. Finally, competition for limiting RISC components at high doses of siRNA can deplete endogenous miRNA pools, indirectly de-repressing their targets and producing "network off-targets" that are mechanistically indirect but biologically real. These layers intersect in vivo, such that the observed signature is a convolution of seed-driven silencing, innate immune remodeling and homeostatic feedback loops.
Off-targeting is intrinsically sown by the very thermodynamics that underlie the correct RNAi process. The guide strand loads into Argonaute with its 5′ phosphate in a basic pocket; nucleotides 2–8 (the seed) are draped across the protein surface in a pre-helical groove used to scan mRNA for complementarity. Perfect complementarity outside the seed is not required; a single G-U wobble or even a bulged adenosine can be tolerated if the seed matches well. Thus, hundreds of transcripts containing a hexameric match in their 3′ UTR can be recruited into the RISC, where they are not cleaved (because they lack full complementarity) but are deadenylated and decapped through recruitment of TNRC6 and CCR4/NOT complexes—precisely the fate of microRNA targets. A second, rarer mechanism involves partial complementarity in the central region (positions 9–12) along with a non-complementary seed; here, AGO2 can still cleave the phosphate backbone, generating a nicked mRNA that is recognised by exonucleases and degraded beyond repair. A third layer is added by passenger-strand loading: if the thermodynamic asymmetry of the duplex is suboptimal, the intended passenger (originally discarded) can itself seed and become a guide, initiating an entirely separate off-target network. Finally, high concentrations of any siRNA will saturate Exportin-5 and TRBP, reducing the availability of endogenous pre-miRNAs and indirectly derepressing their targets—a phenomenon known as "miRNA pathway saturation" that masquerades as sequence-specific toxicity. Together, these mechanisms ensure that off-target effects are not an occasional nuisance but an emergent property of any sufficiently abundant siRNA.
Seed-mediated off-target effects can also dominate over the intended phenotypic signature in cell-based screens and lead to potentially years of wasted target validation. A simple example is a duplex that targets a mitotic kinase, but also targets an anti-apoptotic transcript that is present in the screening model. The apparent "specific" cell-cycle arrest phenotype will be lost when the seed sequence is mutated. Similar to cell-based screens, the results of animal experiments can also be misleading. Targeting of genes encoding hepatocyte metabolic enzymes can modulate drug clearance, leading to an unapparent but increased systemic exposure until toxicities arise, incorrectly ascribed to the intended therapeutic target. Unintended silencing has led to the discontinuation of late-stage programmes on clinical hold, including loss of vision in ocular angiogenesis trials because of unanticipated targeting of neuroprotective genes, and immune suppression in oncology studies because of the off-target knock-down of components of cytokine signalling. As a result, regulatory agencies are now requiring whole-transcriptome and Ago2-CLIP sequencing to define all transcripts that co-purify with the administered guide strand, and a single unpredicted seed-mediated interaction is cause for clinical hold if the target gene is in a pathway with no functional redundancy. Off-target effects are now an integral part of risk-benefit assessment, and have led to a move in the industry towards chemically modified and asymmetric duplexes that destabilize the seed region, as well as delivery vehicles that limit systemic exposure and restrict treatment to the target tissue so that even if off-target transcripts are silenced, the biological consequences are limited.
The evolution of RNAi from bench-side promise to clinical practice has been less driven by identification of new siRNA sequences to target but by reconceptualization of the delivery vehicle. Initial approaches employed simple cationic lipids to collapse the anionic duplex into a particle of less than 100 nm; while such formulations mediate endosomal uptake they also rapidly induce clearance, complement activation and liver sequestration. Current approaches therefore consider the delivery problem as one of multi-scale optimization. At the angstrom scale, the siRNA itself is modified to have a sugar-phosphate backbone that is nuclease resistant while still matching the narrow catalytic cleft of Argonaute. At the nanometre scale, carriers are decorated with pH-switchable lipids that respond to the acidity of endosomes, eject stealth polymers and expose membrane-fusogenic tails that breach the vesicle before lysosomal degradation can occur. At the micrometre scale of tissues, surface topology and ligand density are optimized to take advantage of chaotic tumour vasculature or sinusoidal fenestrations to favour extravasation at the desired sites while sparing healthy parenchyma. Finally, at the scale of the organism, pharmacokinetic modelling is used to combine renal filtration thresholds, macrophage uptake rates and hepatobiliary transit times to determine how frequently a dose must be repeated before silencing plateaus. The smartest systems are therefore not single breakthrough materials, but hierarchically integrated platforms in which each hierarchical layer compensates for the liabilities of the one below it, to collectively transform a fragile nucleic acid into a drug that can be scheduled, dosed, and, if necessary, stopped.
Sequence-level precision is achieved by first accepting Watson–Crick pairing as necessary but insufficient for exclusive cleavage. The first refinement is positional steric tailoring, where the 2′-hydroxyl is exchanged for small hydrophobic groups (fluoro, methoxy, etc.) throughout the seed region (positions 2–8). This increases the enthalpic mismatch cost without changing global A-form helix geometry which is determined by the ribonuclease domain. The substituents shift sugar pucker towards the C3′-endo conformation, discriminating against off-target transcripts that require flexible backbone accommodation; at the same time the fully matched target is maintained in a tight complex. A second refinement is at the phosphodiester linkage itself: partial replacement with phosphorothioate stereoisomers creates a chiral center, and slows exonuclease progression. Only the Sp configuration is tolerated by Ago2; random racemic mixtures dilute the active population and must be enriched during solid-phase synthesis. A third refinement is at the nucleobase itself: 5-methylation of uridine or N4-acetylation of cytosine increases melting temperature by ~2 °C per modification. This allows shorter duplexes that still satisfy the thermodynamic threshold for RISC loading but have fewer seed matches across the genome. Critically, these changes are placed asymmetrically—confined to the passenger strand or to positions 14–19 of the guide—so that the catalytic cleavage site (between bases 10 and 11) is unperturbed and exhibits full slicing competence. A final safeguard is photocaging, where nitroveratryl or bromohydroxycoumarin adducts at selected 2′ positions render the molecule transcriptionally inert until brief ultraviolet exposure. By combining orthogonal chemistries—each tuned to a different vulnerability of off-target recognition—the modified siRNA becomes less like a promiscuous oligonucleotide and more like a proteinaceous enzyme whose active site is sterically gated against imperfect substrates.
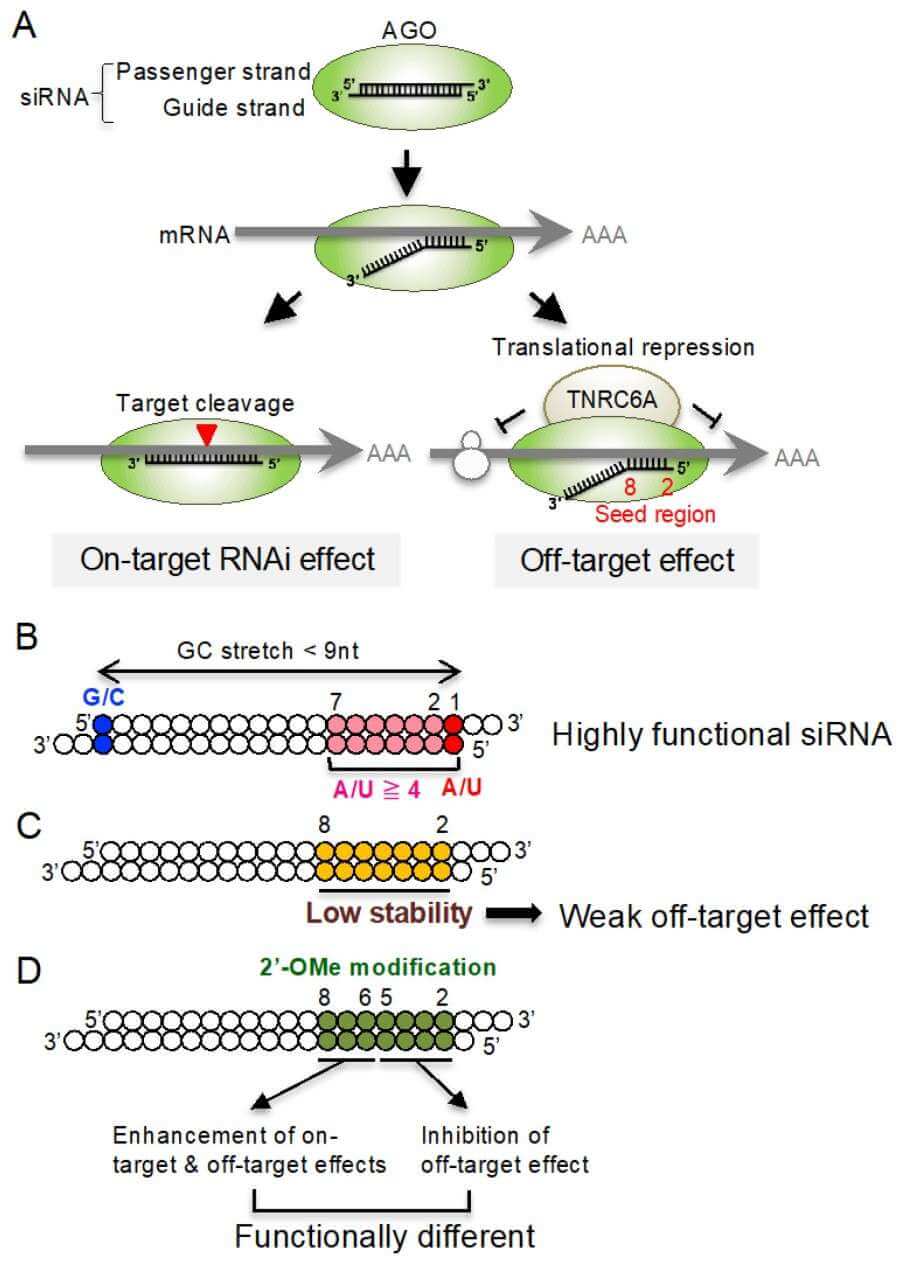 Fig. 1 Mechanism of siRNA-mediated on-target RNAi and off-target effects.1,2
Fig. 1 Mechanism of siRNA-mediated on-target RNAi and off-target effects.1,2
Spatial confinement is provided by presenting the carrier as a modular chassis whose exterior can be tailored independently of the cargo-stabilizing backbone. A common strategy is to use the zwitterionic lipid bilayer, with its outer leaflet graft-engineered with a dense brush of poly-hydroxyl polymers; these polymers stave off opsonins for long enough for the particle to reach the target tissue, but are shed in the acidic tumor interstitium, revealing cationic lipids underneath that in turn promote binding to negatively charged tumor cell membranes. High-affinity ligands can be conjugated to the brush to achieve molecular rather than just physicochemical selectivity, where the choice of ligand is often a peptide, antibody fragment or aptamer, and the conjugation is achieved through maleimide–thiol or strain-promoted azide–alkyne linkages. Ligand density is kept below the theoretical saturation limit so as to avoid multivalent clustering, which would result in accelerated clearance through scavenger receptors. Internally, the siRNA is ionically bound, not just encapsulated, to tertiary amine groups on a biodegradable polymer scaffold; this pre-complexation both prevents leakage in circulation but also allows for release once protonation of the amines in late endosomes disrupts the electrostatic interactions. An additional embellishment is to include fusogenic peptides that change conformation from a random coil to an α-helix with falling pH, which perturb the endosomal membrane and forms transient pores large enough for the siRNA–polymer complex to diffuse into the cytosol before lysosomal fusion. The carrier itself is designed to be subject to renal or hepatobiliary clearance within a certain window: if lipid species whose hydrocarbon tails contain ester linkages susceptible to esterase are used, the particle shrinks to below the cut-off for glomerular filtration, which in turn avoids chronic accumulation in tissues and the chronic inflammation that has dogged earlier non-degradable polymer systems. The end result is a carrier that sequentially traverses vascular, interstitial and intracellular barriers to leave its siRNA payload only with cells that express the target receptor, while leaving receptor-negative bystanders unharmed.
Classic filtering pipelines use BLAST-like alignments to seed candidate sites, followed by manual filtering by thermodynamic scores. The former scales poorly as transcript catalogues increase in size, while the latter penalizes dense off-target landscapes by ignoring the cooperative effects of multiple weak sites. Modern machine learning architectures frame specificity prediction as a high-dimensional classification problem, in which each guide strand is projected into a feature space that includes seed frequency, local GC content, secondary-structure accessibility and cell-type-specific expression quantiles. Gradient boosted ensembles trained on public cleavage maps can learn non-linear combinations of these features to outperform traditional heuristics by recognizing, for example, that a G-U wobble at position 6 is acceptable when the flanking region is AU-rich but unacceptable when embedded in a stem-loop. Generative adversarial networks take it one step further by synthesizing completely new sequences that maximize the margin between on-target knock-down and the cumulative repression of the top predicted off-targets; the discriminator network is incentivized to detect residual liabilities, forcing the generator to explore chemical spaces that human designers rarely try. Because wet-lab validation remains rate-limiting, active-learning loops bias the 2 % of in silico candidates whose uncertainty indices are highest to ensure that each synthesis round maximizes information gain. Reinforcement learning has been co-opted to co-optimize the sequence and chemical modification pattern simultaneously: the agent is given a positive reward when a locked nucleic acid at position 4 suppresses off-target cleavage without diminishing on-target potency, and a negative reward when 2′-fluoro at position 8 induces RIG-I signalling. Over thousands of simulated episodes the policy converges on modification grammars that bench chemists can then synthesize and test. Crucially, the latent representations learned by these models are transferrable across genes and even across species; once trained on a sufficiently diverse corpus, the network can predict the behavior of a hepatic siRNA in renal tissue by re-weighting the expression priors without retraining the entire architecture. By embedding iterative learning inside the design cycle, AI systems are compressing years of empirical optimization into weeks, yielding sequences whose off-target landscapes are sparse enough to meet emerging regulatory thresholds while still retaining the robust knock-down required for therapeutic efficacy.
An siRNA that goes from acting genome-wide to transcript-specific also moves through a drug development process that changes. If a molecule's value comes only from increased potency and selectivity, its economics may look like other therapeutic agents that have been through the process. However, when a product's value creation is sliced and diced into specific categories, it becomes evident that each segment accrues some level of benefit from a molecule that is specific to its target transcript only.
Regulatory agencies have woken up to the fact that, when off-target transcript knock-down can be demonstrated to be below the limit of biological variability, extensive second species toxicology can be eliminated. The regulatory package of seed-inert sequence verification, tissue-specific exposure and transient PK would allow replacement of the chronic dog package with a streamlined 28-day rodent package and transcriptomic monitoring, reducing pre-clinical spend by 10-20% and IND-enabling schedules by 1-2 quarters. The fact that the precision carriers degrade into fragments small enough for renal clearance obviates the need for long term biodistribution studies which previously required radiolabelled lipid monitoring - this being replaced with mass-balance recovery in urine, reducing animal numbers still further. Phase-I entry can also be accelerated - starting doses can be escalated more aggressively if the predicted no-effect level for off-target genes is above the first observable PD response, shortening dose-ranging and smoothing the path into patient cohorts. Approvals conditional on surrogate transcript knock-down would also become possible, when regulators are persuaded that off-target gene silencing is below the limit of statistical detection, thus providing a speedier route to market for ultra-rare indications where clinical endpoints are otherwise unachievable with a practical trial size. In short, precision allows safety to be used to speed development rather than slow it down.
A molecule with chemically gated activity against mismatches is less sensitive to slight variations in process. Minor fluctuations in lipid-to-RNA stoichiometry, leftover ethanol or ionic strength that may have previously altered the off-target signature (thus requiring a narrow release window) are no longer a concern because the altered guide strand will not bind to sub-optimal transcripts. This can lead to improved batch yield, reduced lot rejections, and allows for easier scaling in single-use bioreactors with streamlined analytical methods: high-resolution MS replaces tedious cell-based potency assays, reducing QC release time from weeks to days. Shelf-life specifications can also be relaxed; photocaged or locked-nucleic-acid-containing duplexes are thermodynamically labile, but the chemically gated discrimination is not, which opens the door to room-temperature supply chains in low resource environments without the cold-chain capex. Contract manufacturers can, therefore, bid more competitively, while originators can still have some negotiating leverage because the know-how needed to replicate the exact pattern of orthogonal chemical modifications is non-trivial, creating a technical moat that complements the legal exclusivity. All in all, the cost-of-goods can decrease even while complexity increases, inverting the traditional paradigm that precision and expense go hand-in-hand.
There is an up-front budget impact caveat to conventional oligonucleotide therapies: the co-medications required to dampen thrombocytopenia, the quarterly liver-panel monitoring, the extended hospital stays needed to titrate dose against toxicity. By obviating these supplemental costs, the premium price of a highly precise siRNA can be eclipsed by the offset in the total cost-of-treatment equation, which in this respect is a calculation that health-technology assessment (HTA) organizations are now coming to accept. Even more importantly, if transcript-selective knock-down can be shown in circulating mRNA, this can serve as an immediate surrogate endpoint. Outcomes-based reimbursement contracts are therefore feasible: if the targeted biomarker fails to fall below a pre-determined threshold, the payer is reimbursed. These types of contracts expedite market access (because the financial risk is shared) while also reassuring physicians that they are not simply substituting an untreated disease state for an iatrogenic one. In competitive therapeutic categories (lipid disorders, for instance), precision can be the key differentiator that distinguishes a new siRNA from statin-generic pricing pressures, thus achieving formulary placement at innovative-tier reimbursement even if the LDL-lowering magnitude is not materially greater than that of legacy modalities.
At BOC Sciences, we help biopharma innovators move beyond the limitations of conventional siRNA delivery. Off-target effects, low specificity, and unpredictable gene silencing outcomes can undermine even the most promising RNA therapeutic programs. Our precision-engineered siRNA delivery platforms are designed to eliminate these obstacles-delivering smarter, safer, and more consistent therapeutic performance.
Our approach to siRNA delivery is built on scientific rigor and intelligent design. We combine chemical modification strategies, smart nanoparticle architectures, and targeted delivery technologies to enhance both efficacy and specificity. Our capabilities include:
Each project is tailored to the molecular target, indication, and route of administration, ensuring the highest possible therapeutic precision.
We support our partners across every stage of the development journey-from initial concept through regulatory-ready validation. Our end-to-end siRNA delivery services include:
This integrated model minimizes development risk and ensures every formulation is optimized for translational success.
Our reputation is built on precision, performance, and partnership. By combining deep expertise in RNA chemistry, nanoparticle design, and analytical development, we provide:
Partnering with us ensures your siRNA program benefits from cutting-edge technology and proven translational know-how.
At BOC Sciences, precision isn't a concept—it's our standard. Our intelligent delivery platforms empower drug developers to achieve target-specific gene silencing with minimal off-target effects, unlocking new potential for RNA therapeutics across diverse indications. Collaborate with us today to engineer smarter siRNA delivery solutions that transform complexity into clinical success.
1. What causes off-target effects in siRNA delivery?
Off-target effects occur when siRNA unintentionally silences genes with similar sequences, often due to incomplete sequence specificity or poor carrier targeting.
2. How can precision engineering minimize off-target risks?
Using AI-based sequence optimization, chemical modifications, and targeted nanoparticle systems can significantly increase gene silencing accuracy.
3. What makes a "smart" siRNA delivery system?
Smart systems use stimuli-responsive carriers or modular nanoparticles that release siRNA precisely where and when it's needed, maximizing efficacy and safety.
4. Why is precision important in biopharma siRNA development?
High-precision delivery ensures reliable gene knockdown, reduces toxicity, and accelerates clinical success rates—critical for drug development pipelines.
References