As of now, the total number of confirmed cases of COVID-19 worldwide has exceeded 72 million, and the number of deaths has exceeded 1.6 million. However, there is still no specific medicine for the treatment of COVID-19 in the world.
But what is worth looking forward to is that the COVID-19 vaccine research and development work carried out at the same time as the COVID-19 treatment drug research and development has achieved good results. A number of COVID-19 vaccines have entered phase III clinical trials worldwide, and clinical phase I/II results have been published, and also preliminarily shows the good safety and immunogenicity of the vaccine.
There are currently multiple technological paths for the development of COVID-19 vaccines. Most of the traditional technology paths are inactivation, attenuation, and protein recombination technologies, and their applications are relatively mature. New technology platforms such as viral vector vaccines and nucleic acid vaccines (mRNA vaccines and DNA vaccines) that have emerged in recent years have opened up new directions for vaccine research and development.
Among them, mRNA vaccines are the third-generation vaccines developed after attenuated vaccines, inactivated vaccines, and subunit vaccines. Because of its unique advantages such as safety, effectiveness, and short R&D cycle, it has won the favor of pharmaceutical companies and a large amount of capital investment and has become one of the main technical routes for the COVID-19 vaccine research and development.
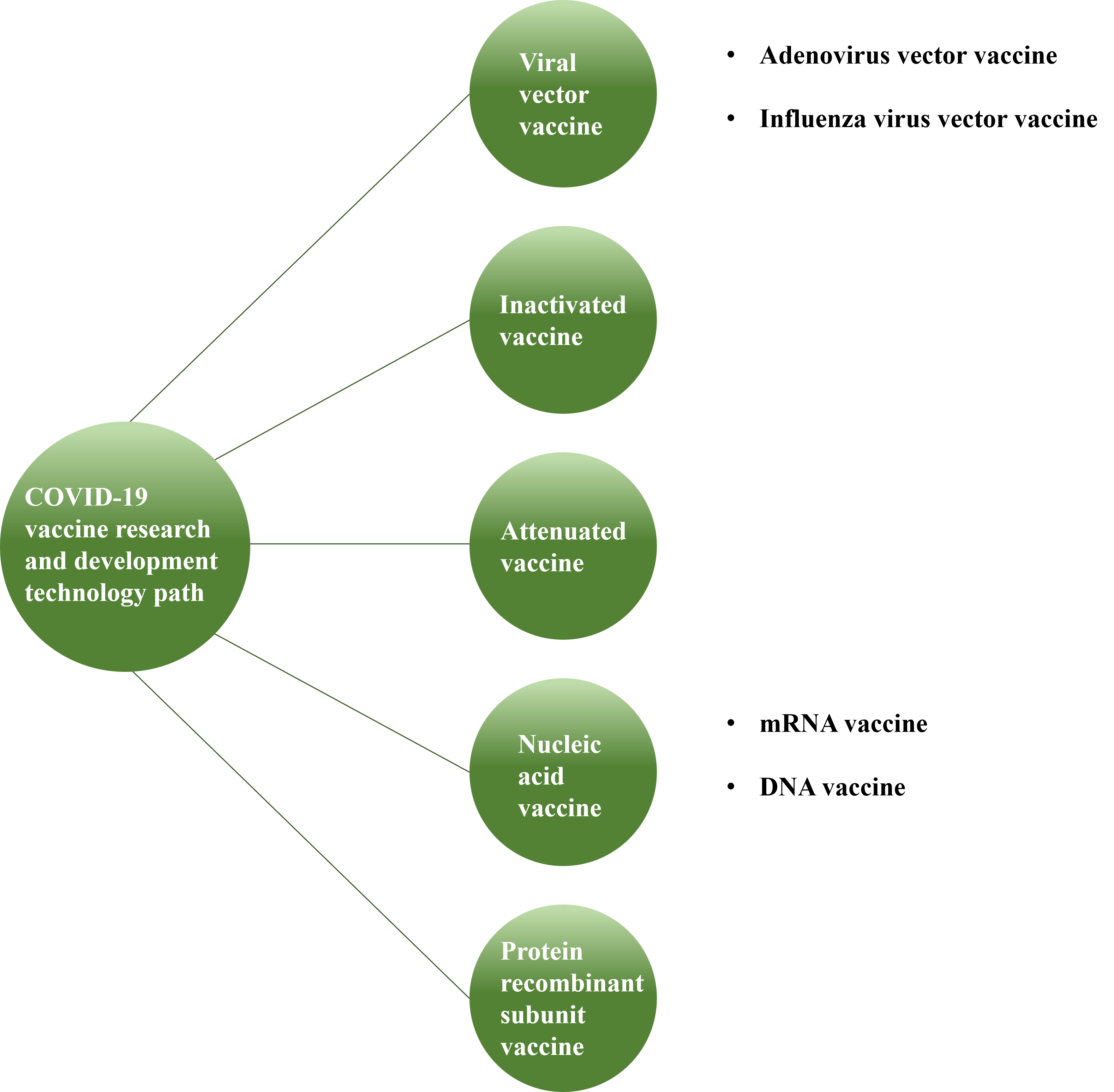 Figure 1: 5 research and development technology paths for the COVID-19 vaccine
Figure 1: 5 research and development technology paths for the COVID-19 vaccine
Unlike traditional vaccines that directly introduce antigen proteins to stimulate the host's immune response, mRNA vaccines are a safer new type of nucleic acid vaccine.
mRNA vaccines transmit the nucleic acid information of antigens into the human body, and the cells inside the body will respond accordingly. The cells inside the body will produce antigens consistent with its information based on this "portrait" to simulate virus infection, and the immune system can then produce an immune response against this. For the COVID-19 virus (SARS-CoV-2), the S protein on the spike is the most critical target antigen.
After vaccination, the mRNA encoding the spike protein (S protein) packaged in a lipid nanoparticle enters the cell and synthesizes the protein in the ribosome. The protein is broken down into smaller fragments (polypeptides) by the cellular proteasome or transported to the outside of the cell through the "transporter" Golgi apparatus.
The antigen peptide fragments enzymatically hydrolyzed in the cell form a complex with the major histocompatibility complex (MHC)-class I protein, which is expressed on the surface of the antigen-presenting cell and is recognized by the "detective" CD8+ T cells to induce the cell-mediated immune response.
On the other hand, the extracellular spike protein can be swallowed, pinocytosed, and broken down into smaller polypeptide fragments by different immune cells, forming complexes with MHC-II proteins, and expressed on the surface of antigen-presenting cells, recognized by CD4+ T cells and promote B cells to produce antigen-specific antibodies.
The mRNA vaccine synthesis and production process is relatively convenient. It can be completed within 1-2 months from vaccine design to sample preparation. The research and development cost is relatively low, and it is convenient for rapid capacity expansion.
The mRNA vaccine is produced through a cell-free in vitro transcription process. Compared with traditional vaccines, its production is easy and fast. In the case of the COVID-19 epidemic, the mRNA vaccine development cycle is expected to be shortened to about 1 year
mRNA will not be integrated into the genome, can be expressed transiently, metabolized and eliminated by the body's natural mechanism, will not persist for a long time, and can avoid therapeutic mutations
mRNA has a certain self-adjuvant effect, can effectively activate the immune response, can produce more comprehensive humoral immunity and cellular immunity, and fight the virus through dual mechanisms
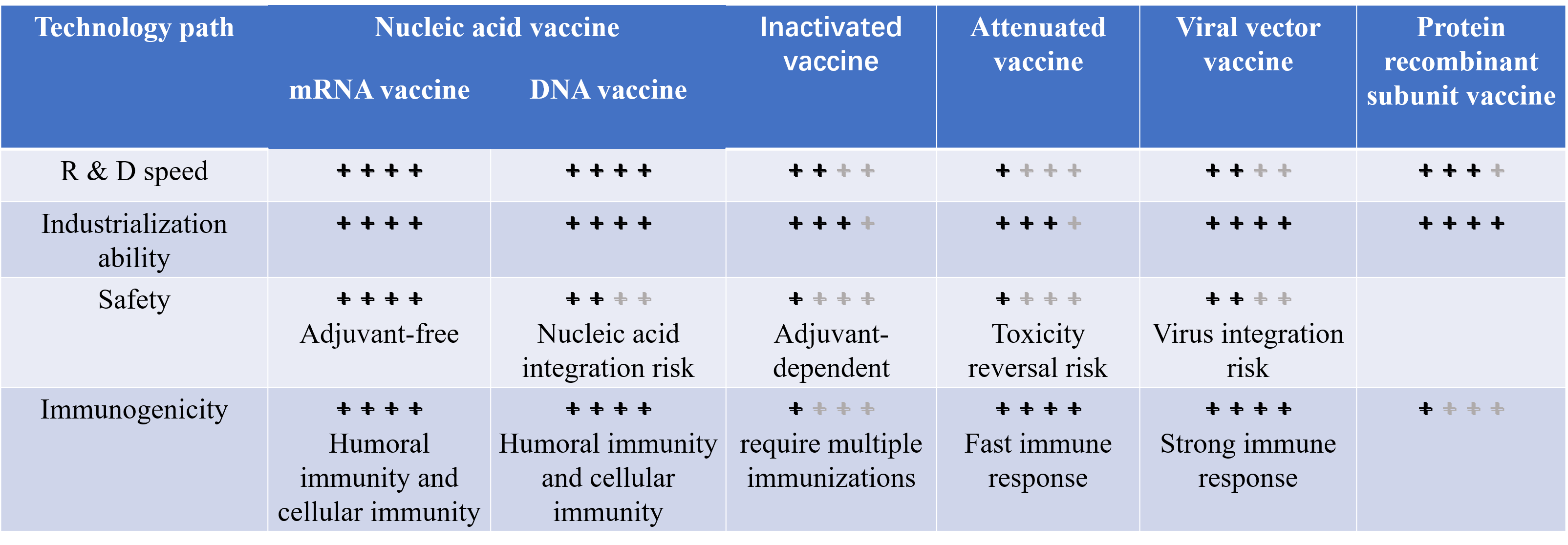
Table 1: Comparison of the characteristics of 5 types of vaccines
mRNA vaccines for COVID-19 in clinical development
As of October 29, 2020, the WHO COVID-19 vaccine progress sketch shows that a number of mRNA vaccines have entered clinical trials (see the table below), including the mRNA-1273 vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases (NIAID) and The BNT162b2 vaccine jointly developed by BioNTech, Pfizer and Fosun Pharma has entered Phase III clinical trials and is currently progressing smoothly.
| Developer/ Manufacturer | Candidate vaccine | Candidate vaccine type | Regulatory status/Trial ID | |||
| Phase Ⅰ | PhaseⅠ/Ⅱ | PhaseⅡ | Phase Ⅲ | |||
| Moderna/NIAID | mRNA-1273 | Lipid nanoparticle (LNP)-encapsulated RNA | NCT04283461 | NCT04405076 | NCT04470427 | |
| BioNTech/Fosun Pharma/Pfizer | BNT162 | 3 LNP-mRNAs | NCT04368728 | ChiCTR2020-001038-36 | NCT04368728 | |
| CureVac AG | CVnCoV | mRNA | NCT04449276 | NCT04515047 | ||
| Imperial College London/Morningside Ventures | LNP-nCoVsaRNA | Self-amplifying RNA vaccine | ISRCTN17072692 | NCT04480957 | ||
| Arcturus Therapeutics/Duke-NUS Medical School | ARCT-021 | Self-replicating RNA and nanoparticle delivery system | ||||
| Academy of Military Medical Sciences, Suzhou Abogen Biosciences and Walvax Biotechnology | ARCoV | mRNA | ChiCTR2000034112 | |||
However, two major challenges related to the immunogenicity and stability of mRNA vaccines must be overcome to make it a viable clinical alternative to conventional vaccines.
These advances have made mRNA vaccines more widely used.
It is not clear which candidate COVID-19 vaccine will make it to the market first, and which vaccine approach will be most effective in the long run. However, clinical trial evidence to date shows that mRNA vaccines have great potential to become a new platform that is fast, safe, and efficient.
Related Articles
mRNA vaccines offer rapid development cycles, high production efficiency, strong immunogenicity, and excellent safety profiles without genomic integration risks.
Lipid nanoparticles protect mRNA from degradation, improve cellular uptake, and enable efficient antigen expression for enhanced immune response generation.
We focus on sequence optimization, codon selection, UTR engineering, and structural modifications to maximize antigen expression and immune activation.
Yes, our mRNA platform enables digital sequence design and rapid in vitro synthesis, allowing parallel testing of multiple antigen variants within weeks.
mRNA vaccines demonstrate faster development timelines, flexible manufacturing, and dual humoral/cellular immune activation compared to conventional platforms.
Key challenges include optimizing delivery systems, enhancing stability, and balancing immunogenicity while maintaining safety profiles - all areas we actively address.