At present, Moderna, a biotechnology company specializing in mRNA therapy and vaccines, announced that its macrophage virus (CMV) vaccine (mRNA-647) has completed the registration of the Phase 2 study. In the past 50 years, no vaccine against CMV infection has been approved. mRNA-1647 is the first infectious disease mRNA vaccine to enter Phase 2 clinical trials.
The CMV virus belongs to the herpes virus family. This viral infection is usually mild or asymptomatic and is always lurking in the host cell. When an infected mother transmits the virus to an unborn child, it can cause congenital (at or before birth) CMV infection, which is the main cause of birth defects. About 7% of newborns in the world have congenital cytomegalovirus infection, and about 20% of infected infants will have birth defects including neurodevelopmental disorders.
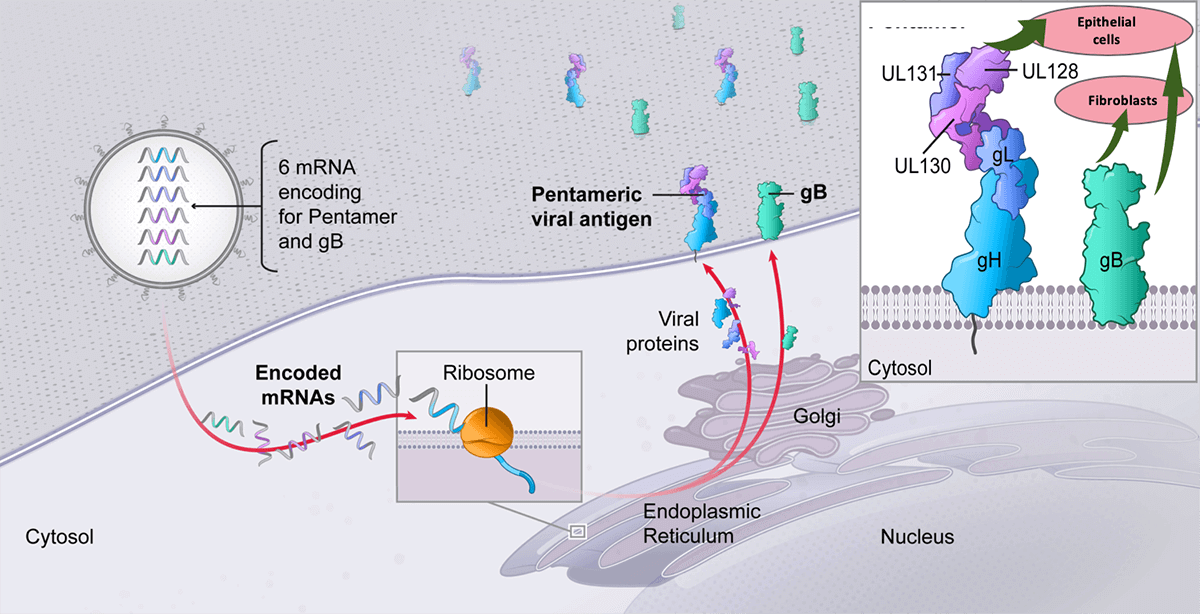 Figure 1: Each CMV vaccine contains 6 mRNAs (5 subunits encoding pentamer complexes, and one encoding glycoprotein B target antigen) (from Moderna's official website).
Figure 1: Each CMV vaccine contains 6 mRNAs (5 subunits encoding pentamer complexes, and one encoding glycoprotein B target antigen) (from Moderna's official website).
mRNA-1647 is a CMV vaccine composed of 6 kinds of mRNA and encapsulated in lipid nanoparticles (LNP). Five mRNAs encode different subunits of CMV and the pentamer complex of the cell membrane, and one mRNA encodes glycoprotein B (gB). These proteins are very important for CMV infection of epithelial cells and fibroblasts. mRNA-1647 is intended to stimulate an immune response to these proteins, thereby preventing CMV infection.
Unlike protein-based vaccines, mRNA-1647 directs cells to specifically construct pentamers and gB antigens, whose structure mimics the structure that the virus presents to the immune system during natural infection. mRNA drugs/vaccines are designed to guide human cells to produce intracellular, membrane or secreted proteins that have therapeutic or preventive effects and have the potential to solve a variety of diseases.
The Phase 2 study is evaluating the safety and immunogenicity of mRNA-1647 in 252 healthy adults. The purpose is to provide a basis for the dose selection in the third phase. The company is actively preparing for the third phase of key research expected to begin in 2021, which will assess the prevention of primary cytomegalovirus infection in populations including women of childbearing age.
As the CEO of Moderna said, "We recognize that women of childbearing age urgently need a preventive vaccine against CMV". Hope that the clinical trial of mRNA-1647 can proceed smoothly and bring good news for CMV infected people!
The BOC RNA platform is built on the scientific basis and application of RNA (mRNA, siRNA, miRNA, dsRNA) and the continuous improvement of delivery technology, providing customers with the ability to find a powerful new development candidate product channel.
* Related Products & Services from BOC RNA
Using multiple mRNAs allows simultaneous expression of several target antigens, enabling complex protein assembly and mimicking natural pathogen structures for more accurate immune or cellular response studies.
Lipid nanoparticles protect mRNA from enzymatic degradation, enhance cellular uptake, and facilitate efficient expression, making experimental results more reproducible and scalable for research applications.
Yes, researchers can design and synthesize mRNA sequences quickly, allowing rapid testing of antigen variants, optimizing expression, and iterating designs without lengthy cloning processes.
Key considerations include codon optimization, balancing expression of each antigen, avoiding sequence interference, and ensuring compatibility with delivery systems to maximize functional protein production.
mRNA platforms enable controlled antigen expression, high-throughput screening of immune responses, and the ability to test multiple targets or formulations efficiently, supporting faster identification of promising candidates.
Products undergo sequence verification, purity assessment, and analytical characterization to ensure stability, reproducibility, and consistent performance in laboratory experiments.