Ribonucleic acid (RNA) is a genetic information carrier that exists in biological cells and some viruses and viroids. Its role in the body is mainly to guide protein synthesis (Fig.1).
 Figure 1. From DNA to mRNA to Protein (Classical genetic central dogma)
Figure 1. From DNA to mRNA to Protein (Classical genetic central dogma)
RNA is divided into messenger RNA (mRNA) and non-coding RNA according to its structure and function; non-coding RNA is divided into large non-coding RNA and small non-coding RNA: large non-coding RNA includes ribosomal RNA, long non-coding RNA, and non-coding RNA. Encoding small RNAs include transfer RNA, ribozymes, small molecule RNAs and so on. mRNA is a type of single-stranded ribonucleic acid that is transcribed from a strand of DNA as a template and carries genetic information that can guide protein synthesis. Messenger RNA (mRNA) was first discovered by researchers in the 1960s and has now become a highly popular eye-catching basic discipline and applied research field. Based on the further understanding of mRNA, from being an intermediate between DNA and protein to thinking that it is a multifunctional molecule that regulates gene function in all life organs, there have been many different types of based mRNA therapy.
In 1990, Jon A. WOLFF and others injected in vitro transcribed mRNA into mice for the first time, and found that it can express activity in mice and produce related proteins in a dose-dependent manner. This method of directly injecting mRNA can produce an immune response by expressing specific proteins. This is the embryonic form of mRNA therapy. In October 2013, Kenneth R Chien’s research group proved that the injection of modified mRNA (modRNA) expressing vascular endothelial growth factor A (VEGF-A) into mouse cardiomyocytes can promote progenitor cell expansion and directed differentiation. VEGF-A modRNA significantly improves the cardiac function of experimental mice and prolongs the survival period of mice by directing the differentiation of epicardial progenitor cells into cardiovascular cell types. This study proves that modRNA is an effective expression sideway. This work is the first to use mRNA as a genetic medicine to carry out cell fate-related therapies. There are currently a variety of mRNA-based cancer immunotherapies and vaccines undergoing relevant clinical trials. Recently, clinical studies have evaluated the therapeutic effects of mRNA therapy on various diseases including hemophilia, sensory nerve disorders, congenital lung disease, cancer, and liver and lung fibrosis. In 2009, mRNA was first used in humans for immunotherapy of cancer. In the 1990s, mRNA drug experiments have involved various clinical applications, including protein replacement, infectious disease vaccines, and cancer. The 2020 new crown epidemic has enabled a batch of mRNA vaccines to be clinically verified, accelerated the investment in mRNA drug technology in various countries, and greatly promoted the development of related areas of mRNA therapy (Fig.2).
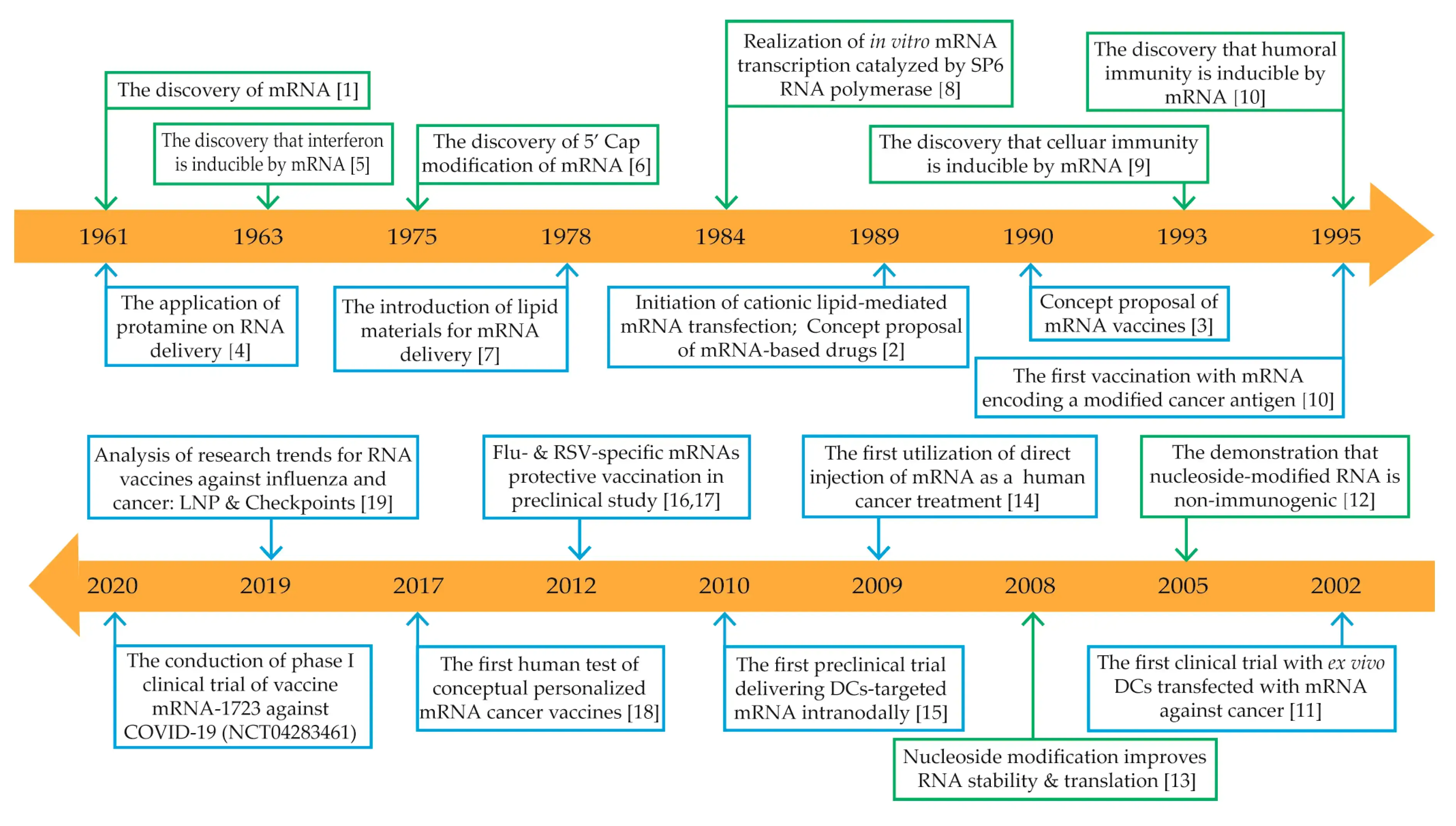 Figure 2. The development history of mRNA therapy (Y Weng, 2019)
Figure 2. The development history of mRNA therapy (Y Weng, 2019)
Compared with DNA therapy and protein therapy, mRNA can theoretically express any protein, so it can be used to treat almost all protein-based diseases. mRNA therapy is more accurate in practical applications and can be used for individualized treatment, compared with current therapies, The production of mRNA is more economical, safer, faster and more flexible. Limitations of DNA therapy: the risk of genome insertion, permanent changes in genes, and difficulty in introducing the nucleus; limitations of protein therapy: difficult protein expression in the body, complex production processes, and strong antigenicity of foreign proteins (easy to degrade). For the characteristics of mRNA not stable enough and short half-life, researchers are currently actively looking for a variety of methods to enhance mRNA stability and extend the half-life, and have achieved certain results.
 Figure 3. mRNA drug development and production process
Figure 3. mRNA drug development and production process
Compared with traditional vaccines, mRNA vaccines have a simple production process, fast development speed, no need for cell culture, and are low cost. Because the mRNA drug research and development process is similar, high-throughput screening can be carried out during the research and development, and the research and development speed is fast; the mRNA drug production process can be directly amplified to quickly produce the drug.
mRNA is a single-stranded RNA molecule transcribed from DNA that carries genetic information to guide protein synthesis, making it a key tool for gene expression studies and synthetic biology applications.
Both standard mRNA and chemically modified mRNA (modRNA) can be produced, enabling enhanced stability, reduced degradation, and improved efficiency in experimental assays.
Strategies include nucleotide modifications, optimized 5' cap structures, poly(A) tail engineering, and sequence optimization to reduce susceptibility to ribonucleases and extend functional half-life.
Challenges include controlling secondary structure, minimizing immunogenic motifs, achieving high yield and purity, and ensuring accurate translation efficiency in downstream applications.
mRNA can be used for cell programming, functional genomics, high-throughput screening, reporter assays, and synthetic circuit construction in molecular biology and biotechnology research.
Yes, BOC RNA provides tailored mRNA design, synthesis, and optimization services, supporting both standard and modified sequences to meet diverse experimental and industrial requirements.
References