On August 10, 2018, the US FDA approved Alnylam Pharmaceuticals' first RNA interference-based therapeutic drug Onpattro (Patisiran) for the treatment of peripheral neuropathy caused by hereditary thyroxine-mediated amyloidosis (hATTR) (Polyneuropathy). In fact, news about the approval of the drug has spread throughout the biotechnology community.
hATTR amyloidosis is a rare genetic disease caused by the misfolding or deformation of a protein called thyroxine protein (TTR) formed in the liver. It is characterized by abnormal aggregation of amyloid in peripheral nerves, heart, and gastrointestinal tract. It is estimated that the disease affects approximately 50,000 people worldwide.
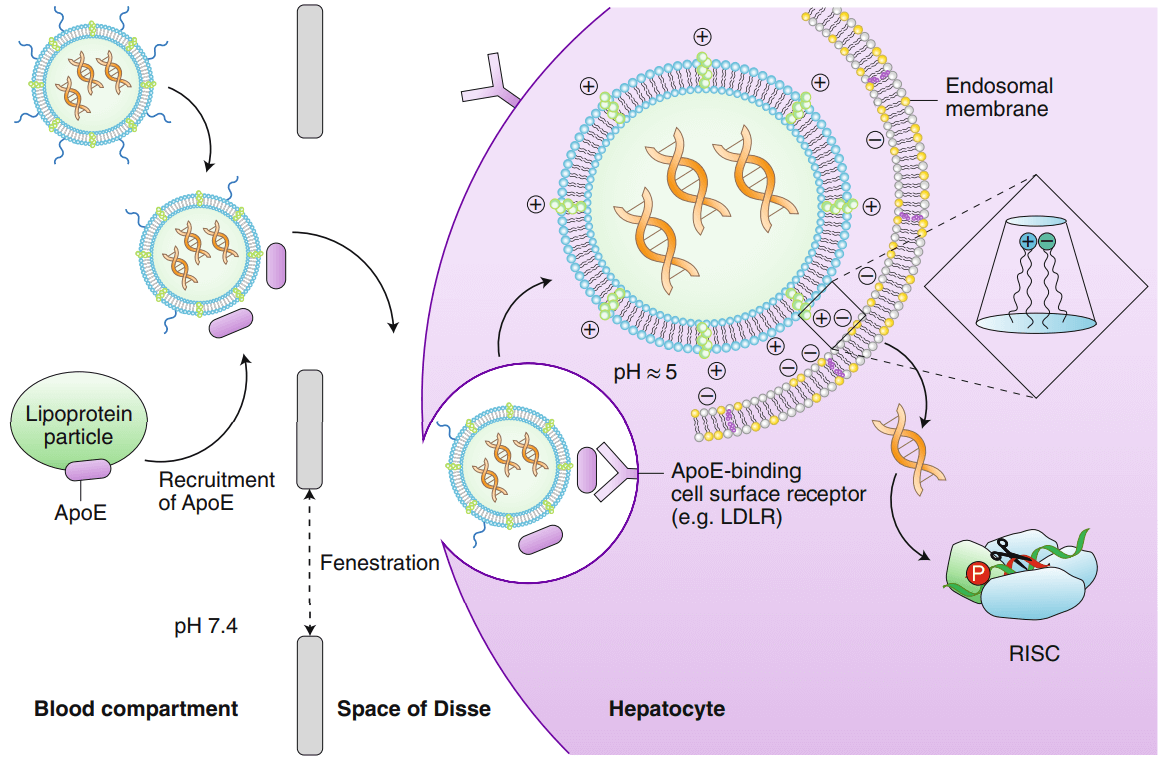 Figure 1: Schematic diagram of lipid nanoparticle (LNP) -mediated delivery of siRNA to hepatocytes in vivo (Akinc, A. 2019).
Figure 1: Schematic diagram of lipid nanoparticle (LNP) -mediated delivery of siRNA to hepatocytes in vivo (Akinc, A. 2019).
Onpattro is a double-stranded small molecule interfering ribonucleic acid (siRNA) formulated in the form of a lipid complex, which is injected intravenously to deliver drugs directly to the liver and targeted to silence the thyroxine transporter (TTR), thereby reducing The level of TTR protein produced in the liver and reduces the deposition of amyloid in tissues.
Onpattro can help reduce the accumulation of amyloid deposits in peripheral nerves by preventing the production of TTR. This can help improve symptoms and help patients better control their condition. Dr. Billy Dunn, director of the Neurology Products Division of the FDA's Center for Drug Evaluation and Research, said: "This unique targeted therapy provides innovative treatments for these patients' symptoms.
Onpattro is the research achievement of the past two decades and is an important milestone, paving the way for the clinical transformation of LNP nanomedicines containing nucleic acid-based drugs, thereby enabling many new therapies based on silencing or expressing target genes.However, the widespread application of RNAi technology still has many challenging scientific problems.
The main technical problem to be solved is how to safely deliver the siRNA to the correct location. Transferring siRNA to specific organs means protecting it from blood degradation and kidney or liver filtration. Depending on different target sites, different delivery methods with a higher therapeutic index are needed. However, the rapid development in recent years shows that it is only a matter of time before these challenges are overcome.
BOC RNA provides you with professional RNAi services, built on the scientific foundation and continuous improvement of siRNA applications, providing customers with the ability to find a strong pipeline of RNAi product candidates.
* Related Products & Services from BOC RNA
Lipid nanoparticles protect siRNA from degradation, facilitate targeted cellular uptake, and improve reproducibility in gene silencing experiments across various cell types or tissue models.
Key considerations include target site accessibility, off-target minimization, GC content, and thermodynamic stability to maximize silencing efficiency and reduce synthesis failures.
Yes, RNAi libraries enable rapid screening of multiple genes simultaneously, allowing functional genomics studies, pathway analysis, and identification of regulatory targets in diverse systems.
Delivery method affects uptake efficiency, cellular localization, and expression outcomes. Choosing between LNPs, liposomes, or conjugates depends on cell type, experimental goals, and scalability needs.
Products are verified for sequence accuracy, purity, and integrity, ensuring consistent performance in experiments and reproducibility across research batches.
Custom synthesis allows rapid design, sequence optimization, and delivery-ready siRNA production, reducing iteration cycles and enabling faster validation of gene function or target interactions.
Ref: