Gold nanoparticle-siRNA_MT1
BRP-02364-X
Gold nanoparticle-siRNA_MT1 refers to the use of gold nanoparticles (AuNPs) as carriers to deliver siRNA (small interfering RNA) targeting MT1 into specific cells or tissues, with the aim of silencing genes and regulating specific biological processes.
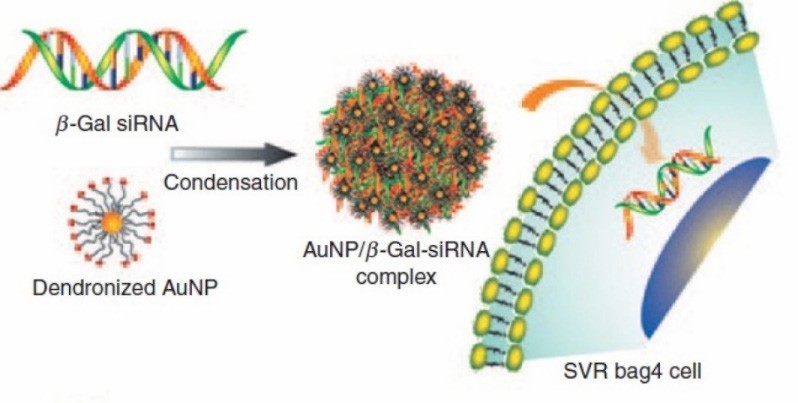 Schematic illustration of AuNP/ β-gal-siRNA complexation and transfection into cells (Kim, Sung Tae, et al, 2012)
Schematic illustration of AuNP/ β-gal-siRNA complexation and transfection into cells (Kim, Sung Tae, et al, 2012)
Gold Nanoparticles (AuNPs) are nanoscale particles made of gold, known for their biocompatibility, ease of functionalization, and ability to facilitate the delivery of nucleic acids. Their surface can be modified with various molecules, including siRNAs, to enhance cellular uptake and target specific tissues.
Small interfering RNA (siRNA) is a class of double-stranded RNA molecules that can specifically target and degrade mRNA, leading to the silencing of gene expression. In this context, siRNA is designed to target the TGF-β1 gene, which is often implicated in cancer progression, fibrosis, and other pathological conditions.
MT1 is a cytokine that plays a crucial role in cell growth, differentiation, and immune response. Overexpression of MT1 is associated with various diseases, including cancer. By silencing MT1, the therapeutic aim is to inhibit tumor growth, reduce fibrosis, and improve overall treatment outcomes.
Studies have shown that binary supercluster complexes composed of gold nanoclusters and siRNA can enhance the anti-tumor immune response of stereotactic ablative radiotherapy (SABR) for primary and metastatic tumors. This complex combines gold nanoclusters as a radiosensitizer and siRNA targeting the immunosuppressive mediator Gal-1, which can promote tumor-inhibitory leukocytes, upregulate cytotoxic granzyme B, and reduce immunosuppressive cell populations, thereby enhancing the radiodynamic and immunotherapeutic effects of SABR on primary and metastatic tumors.
In the process of siRNA delivery, stability is a critical issue that significantly impacts its efficiency and therapeutic effectiveness. Key challenges include nuclease degradation, serum instability, inefficient intracellular release, endosomal entrapment, and immunogenicity. These challenges can be mitigated through strategies such as chemical modifications of siRNA, protective carriers like gold nanoparticles, and stimuli-responsive release mechanisms. Gold nanoparticles can be modified for tumor-specific targeted delivery of siRNA. By conjugating specific oligonucleotides (such as aptamers) on the surface of gold nanoparticles, tumor cell-specific recognition and endocytosis can be achieved, thereby enhancing the precision and efficiency of siRNA delivery while minimizing off-target effects on normal cells. This integrated approach addresses stability concerns and improves the overall potential of siRNA-based therapies. The gold nanoparticles are bound to a user-selected oligonucleotide sequence via a proprietary covalent polymer bridge and are known for their resistance to high temperature, salt and pH changes, as well as good load capacity. These products play an important role in diagnostic, imaging and therapeutic research in the life sciences sector.
In vivo distribution and metabolism of gold nanoparticle-siRNA complexes are critical for delivery efficiency and safety. The distribution is influenced by factors such as particle size, surface modifications (e.g., PEGylation or targeting ligands like aptamers), and administration routes, with optimal sizes (10-100 nm) enhancing tumor accumulation via the EPR effect. Metabolically, siRNA may degrade via nucleases, while gold nanoparticles are slowly oxidized or dissolved, releasing gold ions (Au3+). Clearance occurs primarily through the liver and spleen via the mononuclear phagocyte system (MPS) for larger particles, while smaller particles (<10 nm) are rapidly cleared by the kidneys. Potential toxicity includes immune activation by siRNA and inflammation or organ damage from high doses of gold nanoparticles. Strategies to optimize distribution and metabolism include size control, surface functionalization, stimuli-responsive release mechanisms, and reducing nonspecific accumulation in the liver and spleen. These approaches enhance targeting, stability, and therapeutic efficacy while minimizing off-target effects.
siRNA_MT1 (sequence provided by customer), Diameter (1.8-1500 nm), Oligo Sequence (from 5' to 3', min 10 mer up to 25 mer), Bridge Length (4C, 10C, 20C), Fluorophore Option (550-800nm), Buffer (stable from pH 4-9; 18MEG DI water, PBS, MES, Sodium Borate, TRIS), Volume (1mL, 5mL, 10mL and more), Folic Acid, OD50, Certified Sterilized, Endotoxin Purified, Negative Control, in vivo grade.
Folic acid is conjugated separately to the gold nanoparticles to reduce siRNA interference. With only folic acid labeling, 50% more siRNA will be loaded.
Refrigerator.
Wu, Jindao, et al. Journal of biomedical nanotechnology 12.4 (2016): 800-810.
Nanoparticles, especially gold nanoparticles (AuNPs), have been shown to be an efficient carrier to deliver small RNAs into cancer cells. In this study, we used cysteamine-functionalized AuNPs to effectively deliver TGF-β1 siRNA into hepatoma HepG2 cells in vitro and in vivo. We found that, compared with AuNPs-mediated NC siRNA (AuNP-siNC), AuNPs-delivered TGF-β1 siRNA (AuNP-siTGFβ1) efficiently decreased the level of TGF-β1, increased cell apoptosis, and significantly inhibited the proliferation of recipient tumour cells. Systemic administration of the AuNP-siTGFβ1 complexes into human HepG2 xenografted mice likewise reduced TGF-β1 expression and downstream TGF-β1 signalling. Functionally, AuNP-siTGFβ1 strongly inhibited tumour growth and improved the survival rate of tumour-bearing mice compared with the control groups. In conclusion, our results demonstrate that the siRNA delivery system with AuNP described here appears to be a highly effective method to deliver RNAi therapeutics into tumour cells for oncotherapy.