The distinction between nucleosides and nucleotides is not nitpicking. It's a lever to control reactivity and the fate of impurities and the regulatory category they fall into: nucleosides (base + sugar) are the building blocks of the amidites, and nucleotides (base + sugar + phosphate) are the triphosphate substrates. If you ignore the distinction, you can find yourself misclassified for GMP, with different stability requirements and failed technology-transfer. But if you respect the division, it becomes possible to let the developers pump the nucleosides into solid phase synthesis and the nucleotides into enzymatic transcription, under different but compatible quality regimes.
A nucleoside is a base-plus-sugar molecule without a phosphate. A nucleotide is the same nucleoside esterified with at least one phosphate; this single functional group difference determines whether the molecule is delivered as a protected monomer for phosphoramidite chemistry or as a pre-activated triphosphate for enzymatic polymerization, and thereby drives manufacturing logistics and quality specifications.
A nucleoside is missing the 5'-phosphate so is delivered to solid-phase chemistry as a phosphoramidite building block that will be converted to phosphodiester (potentially across a linker to a longer linker in case of reversed-phase chemistry) downstream, whereas a nucleotide has the phosphate already in place and can be used directly in solution-phase enzymatic polymerization or in microfluidic primer-extension based formats. The phosphate status of a nucleoside/nucleotide therefore determines whether anhydrous acetonitrile or aqueous buffers are used as solvents, whether acid-labile DMT or base-labile acyl protecting groups are applied, and whether the analytical chemistry used is IP-RP-HPLC or ion-exchange. Being ionized at physiological pH, nucleotides must have their counter-ions screened for safety and have osmolality adjusted for delivery, whereas nucleoside-based oligonucleotides are neutral until the final oxidation so have a simpler in-process control. The pharmacopeial monographs will also differ between nucleotides and nucleosides, with the former expected to meet residual solvent Class I limits as raw materials while the latter focus on anomeric purity and heavy-metal scavenging because of the very different chemistries involved.
Table 1 Manufacturing Consequences of Nucleoside/Nucleotide Confusion
| Attribute | Nucleoside route | Nucleotide route |
| Starting point | Base + sugar | Base + sugar + phosphate |
| Solvent system | Anhydrous MeCN | Aqueous buffer |
| Protection scheme | DMT + acyl | Base-labile only |
| Impurity focus | Anomer, metal | Residual solvent, endotoxin |
| Final charge | Neutral (pre-oxidation) | Anionic throughout |
To the cell, nucleotides are energy and signalling currency so it maintains triphosphate pools of species that are highly phosphorylated and complexed with magnesium; to the chemical plant, they are crystalline mono- or diphosphate salts that have to be protected from alkaline hydrolysis and divalent-metal catalysed depurination. Polymerases work with 3'-OH nucleotides but chemical synthesizers require 3'-phosphoramidites, so the same "G" moiety is formulated as 2'-deoxy-guanosine-3'-[(2-cyanoethyl)-(N,N-diisopropyl)] -phosphoramidite in organic solvent or guanosine-5'-triphosphate in aqueous Mg²⁺. Regulatory bodies view nucleotides as candidate drug substances so trigger stringent genotoxic impurity limits, but see nucleosides as advanced intermediates and relax the specs. The biological context thus demands cofactor-grade purity and chirality, the chemical context anhydrous stability and control of β-anomerism, and manufacturers end up with two quality systems for what looks like the same genetic letter.
The use of nucleoside phosphoramidites allows for multi-kilogram scale solid-phase syntheses using reusable solid supports, but at the expense of requiring dry rooms, acidic waste treatment and reagents for regeneration of the DMT cation. The use of nucleotide triphosphates instead, allows for aqueous-phase enzymatic polymerisation and can come with lower numbers of protection and orthogonal cleavage steps as well as less organic waste generation, but would require significant ultrafiltration skids to deal with magnesium pyrophosphate contamination, endotoxin free water and consideration of the source of the RNAse-free enzymes. Thus the decision on phosphate protection quickly impacts considerations of the production facility (dry, explosion proof vs. aqueous, biocontained), supply chain (nucleoside vendors are screened for their anomeric purity and potential heavy metal carry-over whereas nucleotide vendors are screened for bioburden and potential phage contamination) and the overall budget, as well as environmental impact and regulatory planning long before any product specific molecule is coupled to a growing chain.
Nucleosides have a heterocyclic base linked to a pentose sugar molecule via an N-glycosidic bond, while nucleotides are modified with one or more phosphate esters at the 5'-hydroxyl; this one structural modification transforms a lipophilic, membrane-permeable metabolite into a highly anionic component that determines solubility, purification and coupling strategy, and regulatory classification during oligonucleotide production campaigns.
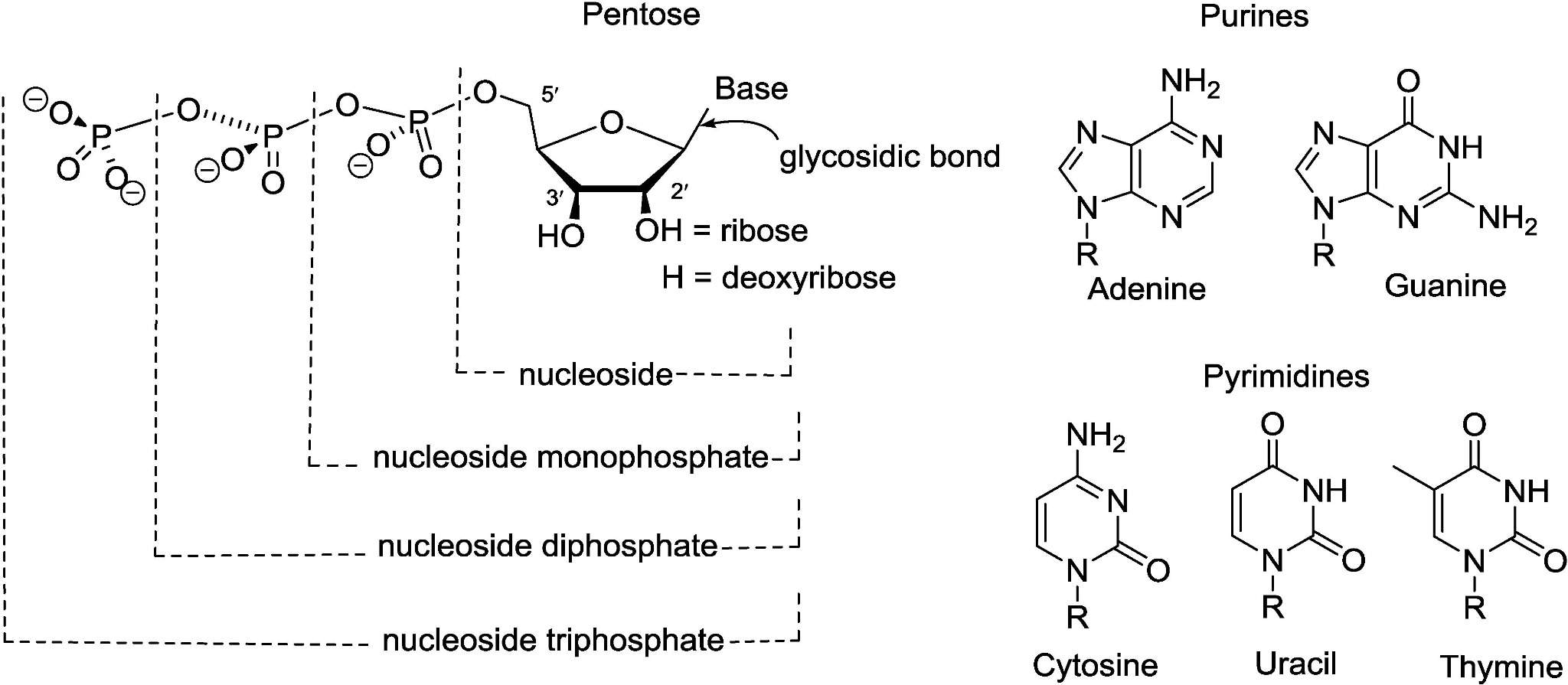 The structural compositions of nucleosides and nucleotides.1,5
The structural compositions of nucleosides and nucleotides.1,5
A nucleoside is a nitrogenous base (purine or pyrimidine) β-N-glycosidically linked to a pentose (ribose or 2-deoxyribose) without a phosphate group. They are uncharged, so hydrophobic and acid-labile, making them compatible with phosphoramidite chemistry (all base protecting groups are acid-removable). The lack of phosphate is important for stepwise solid-phase synthesis, as coupling steps can be performed sequentially without having to deal with premature ion-exchange-related issues. The neutral molecule is also beneficial for HPLC purification of monomers after release, though the absence of phosphate necessitates an additional phosphorylation step for nucleotide products, adding a step with associated new impurities (such as regio-isomeric phosphates) that must be fate-mapped in a regulatory filing.
Phosphorylation adds one, two or three phosphate esters (usually on the 5'-carbon), which renders it a negatively charged, hydrophilic molecule. The phosphate group also creates new reactive centres: the α-phosphate may be stereochemically pure (Sp or Rp) in the case of phosphorothioate drugs, while the γ-phosphate is the leaving group during polymerase-mediated chain extension. Nucleotides are the substrates of kinases and polymerases, making them useful for enzymatic mRNA production, or for radioactive labelling in PK studies. The negative charge means that nucleotides require base-labile protection during solid-phase synthesis, and that different HPLC columns are used (anion-exchange rather than reverse-phase). As a result, mis-ordering a nucleotide instead of a nucleoside results in a complete re-optimisation of analytical methods and salt gradients.
Phosphates impart several new characteristics: they allow for metal chelation (Mg2+, Mn2+) necessary for polymerase catalysis, they introduce chiral centres in phosphorothioate analogues which must be stereocontrolled and they also provide negative charges which change lipophilicity and renal clearance. Cyclic phosphates (cAMP, cGMP) are second messengers, while triphosphates (ATP, CTP, GTP, UTP) are energy currency and polymerase substrates. In the oligonucleotide drugs, the phosphate group also controls linker chemistry: cyanoethyl groups are employed for phosphoramidites while phosphorothioate linkages have sulfurisation steps that generate new degradants (oxidative desulfurisation products) that need to be tracked by LC-MS. In other words, the phosphate is not simply an appendage; it is a switch that rewires reactivity, analytics and biological role.
In the context of biology, nucleotides are used as an energy currency, as signalling ligands and polymerase substrates. In the context of manufacturing, nucleosides are used as oil-soluble phosphoramidite precursors. Understanding this reversal of context is what distinguishes whether the same molecule is regulated as a metabolite or as a chemical intermediate, which then leads to a completely different set of purity specifications, solvent decisions and GMP documentation trajectories.
Intracellularly nucleotides function as energy carriers, allosteric effectors of enzymes and second messengers. ATP is the molecule that is used to "pay" for thermodynamically unfavorable biosynthetic reactions and GTP is used to energize ribosomal translocation and signal-transducing G-proteins. Cyclic nucleotides transmit extracellular signals into transcriptional programs and nucleotide-derived cofactors such as NAD+ and FAD mediate redox flux through the electron-transport chain. Salvage kinases convert nucleosides back into nucleotides so that the cell maintains a non-limiting supply of nucleotides for DNA replication and RNA transcription. Disruption of these pathways causes immunodeficiency, neurodegeneration and cancer.
Table 2 Biological Roles of Nucleotides
| Metabolic role | Nucleotide form | Enzyme partner | Cellular outcome |
| Energy carrier | ATP, GTP | ATPase, EF-Tu | Drive endergonic reactions |
| Second messenger | cAMP, cGMP | PKA, PKG | Signal transduction |
| DNA synthesis | dNTPs | Pol δ/ε | Genome replication |
| RNA synthesis | NTPs | Pol II/III | Transcription |
| Salvage recycling | IMP, UMP | HGPRT, UPRT | Conserve nitrogen/energy |
Solid-phase phosphoramidite synthesis is compatible only with anhydrous solvents and acid-labile protecting groups. The presence of nucleotides (whose phosphates are negatively charged) would result in salt precipitation or ion-exchange related problems. Fully protected nucleosides, which carry acid-labile groups at the 5'-OH and the base nitrogens, are neutral and lipophilic and they can be coupled to high degree in dichloromethane. Oxidation or sulfurisation after coupling allows in situ preparation of the phosphate backbone with controlled stereochemistry at the phosphorothioate linkages. This strategy is free from regio-isomeric phosphate mixture and has been successfully used to produce kilogram amounts of siRNA or antisense oligonucleotides without chromatographic purification, other than two precipitations.
Enzymatic (chemical and physical) synthesis of RNA (recombinant T7 RNA polymerase and NTP substrates) is aqueous, stereospecific, and capable of incorporating modified NTPs such as N6-methyl-ATP or pseudouridine-UTP, with implications for co-transcriptional immune evasion. In comparison, solid-phase (chemical and organic-solvent) synthesis is stepwise, and ideal for synthesizing short antisense or siRNA with specifically placed 2'-O-methyl or phosphorothioate linkages. The two synthetic routes are complementary (enzymatic for long or modified transcripts; solid-phase for short, chimeric oligonucleotides), and selecting an inappropriate substrate class (nucleoside instead of nucleotide) would either stall T7 polymerization or cause the solid-phase column to become clogged with ionic precipitates, making substrate identity contingent on synthetic modality early in the drug development process.
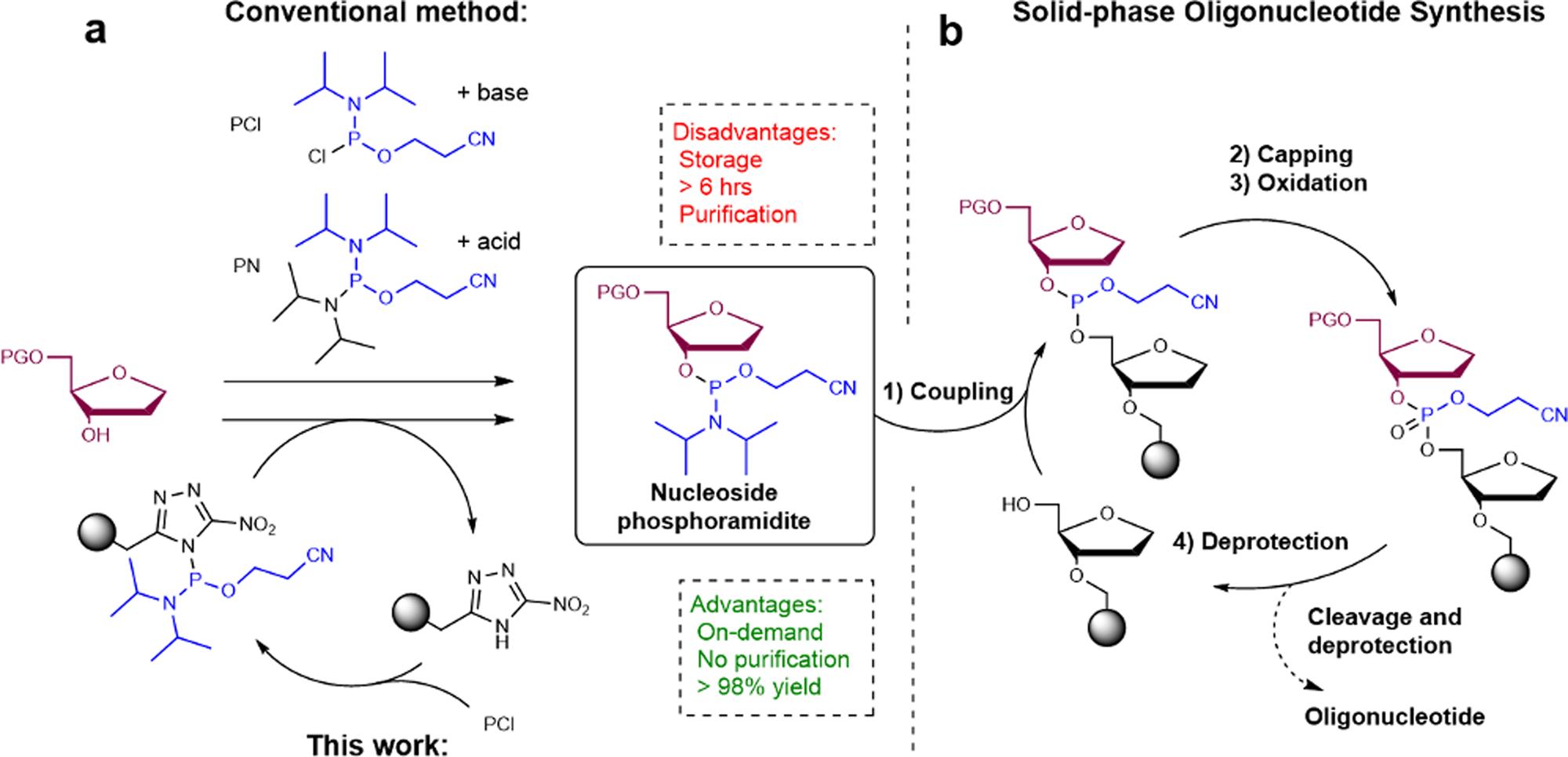 Strategy for phosphoramidite synthesis in a flow-based setup.2,5
Strategy for phosphoramidite synthesis in a flow-based setup.2,5
The reason for making oligonucleotides starting from the nucleosides is that nucleosides are neutral and lack phosphate groups, allowing anchorage to a solid support through acid-labile protecting groups and the use of anhydrous coupling and repeating elongation steps that are not affected by salts. The chemical "canvas" is finally phosphorylated in place to form any desired oligonucleotide. Thus, nucleosides are the building blocks for all siRNA, antisense, mRNA, and aptamers.
The lack of 5'-phosphate eliminates the major site of hydrolysis, so the nucleosides can be long-term stored as lyophilized powders without buffer-mediated depurination. The uncharged core permits stainless-steel reactors and glass-lined vessels without pitting corrosion that would be caused by repeated exposure to anionic phosphate salts. Low hygroscopicity enables weighing and dispensing under typical factory humidity, while the absence of charge averts electrostatic clumping which would otherwise clog pneumatic transfer lines. As a result, multi-kilogram drums can be managed at ambient temperature without inert-atmosphere gloving, simplifying logistics and minimizing occupational exposure controls when compared with nucleotide salts.
The neutral 5'-hydroxyl is protected by a transient dimethoxytrityl (DMT) group that is stable to repeated washes with acid, but removed in seconds by mild dichloroacetic acid, achieving quantitative deblocking without base-mediated transamidation. The exocyclic amines are protected by base-labile benzoyl or isobutyryl groups, which are stable to anhydrous oxidation conditions, but are removed cleanly under aqueous ammonia, to prevent cross-contamination of cycles. The 2'-hydroxyl of ribonucleosides is protected by a fluoride-labile silyl ether, allowing the synthesis of chimeric RNA/DNA strands whose lipophilicity can be switched on demand. Since there are no phosphate protonation equilibria, protection efficiencies are >99 % for adenosine, guanosine, cytidine and thymidine scaffolds, leading to uniform cycle kinetics for automated synthesis.
The final conversion adds 2-cyanoethyl-N,N-diisopropyl-phosphoramidite to the 3'-hydroxyl via phosphitylation under anhydrous conditions. No racemization occurs because the vicinal 2'-substituent cinches the furanose ring, delivering only the β-anomer required for Watson–Crick fidelity. The solid phosphoramidite is isolated by crystallization from acetonitrile/hexane which removes excess reagent and trace water in a single filtration step. The cyanoethyl group is a temporary phosphate protecting group which auto-cleaves upon mild basic oxidation, thus avoiding an extra deprotection step. Once bottled under argon, the solid amidite is stable for years and can be dissolved just-in-time into anhydrous acetonitrile for automated coupling cycles that scale from milligram to multi-kilogram production of oligonucleotide drugs without re-optimization.
Nucleotides are used where the delivery system requires enzymatic specificity instead of chemical versatility, i.e. in RNA-based technologies where polymerases transcribe 5'-triphosphate reservoirs into lengthy single-stranded outputs that resemble endogenous mRNA, replicons that self-replicate or guide sequences for CRISPR; this hydrophilic approach circumvents organic byproducts but necessitates endotoxin, metal and RNase free conditions not required for the solid-phase nucleoside-based strategy.
Table 3 Entry points for nucleotides vs nucleosides in therapeutic workflows
| Application | Preferred substrate | Chemistry | Key quality attribute |
| siRNA/ASO | Nucleoside amidite | Solid-phase | Anomeric purity |
| mRNA vaccine | Nucleotide triphosphate | Enzymatic IVT | Pyrophosphate ≤0.5 % |
| CRISPR guide | Nucleotide triphosphate | T7 transcription | Counter-ion balance |
| Aptamer | Nucleoside amidite | Solid-phase | Moisture ≤0.2 % |
RNA polymerases can recognise each of the four ribonucleoside-5'-triphosphates (NTPs) as substrates, and their incorporation is catalyzed by a two-metal-ion mechanism, which provides Watson–Crick selectivity but with a tolerance for 2'-fluoro, 2'-amino or other base-modified analogues such as N1-methyl-pseudouridine triphosphate. The triphosphate group is used as both energy source and leaving group, so no external activator is required. Reaction buffers typically use Mg²⁺ or Mn²⁺ to help stabilise the transition state. A pyrophosphatase can also be added to hydrolyze the pyrophosphate leaving group and so push the equilibrium towards polymer elongation. As the enzyme copies the template DNA strand-by-strand, sequence errors are trivial, and transcripts with lengths beyond four kilobases can be produced with single-nucleotide precision, a scale currently unreachable by phosphoramidite chemistry. For this reason, nucleotide triphosphates remain the programmable ink of choice for any therapeutic RNA that must preserve native 5'-cap and 3'-poly(A) features while encoding immune-silent base edits.
IVT synthesizes linearised plasmid or PCR-synthesized DNA into gram quantities of capped mRNA in a matter of hours. The reaction mixture consists of a mixture of ATP, GTP, CTP and UTP (or nucleotide analogs) in the presence of recombinant RNA polymerase, ribonuclease inhibitor, pyrophosphatase and a cap analog, for example CleanCap or anti-reverse cap analog; co-transcriptional capping results in 80–95 % capped transcripts, avoiding additional post-synthetic enzymatic processing steps. Self-amplifying RNAs also contain alphaviral replicase open-reading frames that replicate the message, which can reduce the amount of RNA that needs to be delivered. Circular RNA vaccines use IVT to synthesize long precursors that then self-splice under the catalysis of magnesium ions to generate a covalently closed backbone which is protected from exonucleases. In this way, IVT serves as the last-mile technology between the digital sequence of the gene of interest and ready-for-shelf therapeutic RNAs, as long as the nucleotide feedstocks have endotoxin and heavy-metal levels within thresholds for parenteral administration.
Nucleotide triphosphates have stringent triphosphate purity (≥98 %), counter-ion balance (Li⁺, Na⁺, Mg²⁺) and pyrophosphate limits (≤0.5 %) that are necessary to prevent polymerase inhibition during transcription. In contrast, nucleosides are typically controlled for anomeric purity (≥99 %), moisture (≤0.2 %) and residual-metal (ICH Q3D) specifications that are necessary to maintain coupling efficiency of amidites. Due to their hygroscopic nature and propensity to hydrolysis, triphosphates are lyophilised, packaged under nitrogen and stored at −20 °C, while crystalline nucleosides are stable at ambient conditions for ≥24 months; this contrast requires vendors to operate in two parallel quality paradigms: crystalline nucleosides for chemical synthesis and lyophilized nucleotides for enzymatic transcription, with each platform having its own stability pathway, fate of impurities and viral-safety chapter so that the same chemical backbone is controlled by overlapping but non-redundant GMP pathways.
The decision to use nucleosides or nucleotides as starting materials sets the production scheme: the nucleosides provide crystalline, organic soluble intermediates for the phosphoramidite chemistry while nucleotides provide water-soluble, phosphorylated substrates for enzymatic polymerisation. This seemingly small difference results in an overall cost structure, storage and quality control requirements that are vastly different from each other and must be decided before any gram of drug product is synthesized.
A nucleoside synthesis is started by the glycosylation of a heterocycle, under anhydrous conditions, selective protection of the sugar hydroxyls and of the exocyclic amine, the resulting phosphoramidite is then isolated by crystallization. This process skips chromatography and allows for multi-tons campaigns at moderate consumption of solvents. A nucleotide synthesis, on the other hand, needs phosphorylation of the 5'-hydroxyl with POCl3 or an enzymatic kinase and repeated anhydride formation until the triphosphate is obtained. Each of these steps require aqueous work-up, ion-exchange polishing and magnesium removal. This increases the amount of solvents and the complexity of the waste treatment. Nucleotides are charged throughout their synthesis, so losses build up at each phase separation and specialized freeze-dryers are required to get free-flowing powders. This results in a cost curve that is more favorable for nucleosides for long gapmers and nucleotides are acceptable only when enzymatic fidelity is mandatory.
Nucleosides can be considered as simple organic molecules: stable as a crystalline powder in polyethylene-lined drums for years at ≤30 °C and 60 % RH, with only a negligible anomerization in the presence of even small traces of acid catalysts (<0.1 % residual). By contrast, nucleotides are much more sensitive to ambient conditions, because of their labile phosphoanhydride bonds which will hydrolyze even with trace water. To prevent hydrolysis of the anhydride bonds in triphosphates, storage at −20 °C under argon with desiccant sachets is needed to prevent pyrophosphate cleavage. Temperatures of only a few minutes can transform GTP to GDP and inorganic phosphate, which then changes the stoichiometry of enzymatic polymerization and reduces transcript integrity. Mandatory double-bagging, oxygen scavengers and controlled thaw cycles greatly increases warehouse footprint and cold-chain costs. In other words, the phosphate group converts a shelf-stable raw material into a temperature-controlled biological raw material.
The regulatory split is such that nucleosides are considered starting materials (ICH Q11) with the need for anomeric purity, residual-metal (ICH Q3D) and moisture specs but without the viral-safety chapters of the supplements relevant to biologically derived substrates. Nucleotide triphosphates are instead active substrates (ICH Q6A) with the need for triphosphate purity ≥98 %, counter-ion balance (Na+, Li+, Mg2+) and pyrophosphate ≤0.5 %, each of which is tracked by ion chromatography and capillary electrophoresis. Awareness of this regulatory demarcation line permits companies to operate largely separate quality systems for crystalline nucleosides used in chemical oligos and lyophilised triphosphates for enzymatic RNA without duplicative validation efforts, shortening tech-transfer cycles and immunising the pipeline from geopolitical fragilities in the raw-material base.
The choice between nucleoside or nucleotide dictates the parameters for all subsequent stages in drug development. This includes the chemical landscape (dry phosphoramidite chemistry or wet enzymatic synthesis), the nature of the impurities (soluble side-products or pyrophosphate-related degradation products), and a priori GMP record-keeping (synthetic intermediates or actual drug substance), and consequently pre-determines key parameters such as cost, scale-up, and regulatory risk before the first toxicology package is prepared.
Whether DNA or RNA, the first step in the modality-first decision tree for drug development is that, since antisense or siRNA candidates require base-by-base accuracy, the process begins with crystalline nucleosides that are first chemically modified into 5'-DMT, 3'-phosphoramidites that each couple with >98 % efficiency in an anhydrous tetrazole activation system. Such efficiency is required to avoid enzymatic side-reactions and to allow stereopure phosphorothioate or 2'-OMe linkages to be added to the growing chain. In contrast, if the product is self-amplifying RNA or mRNA for a vaccine, then kilogram scale transcription is required and aqueous nucleotide triphosphates (ATP, GTP, CTP, UTP) are fed to T7 polymerase in a single, magnesium-buffered reactor; this same monomer pool may also be spiked with N¹-methyl-pseudouridine or 5-methyl-cytidine to avoid innate sensors. In both cases, the substrate choice pre-defines both the chemistry route and the immunogenicity profile before scale-up.
Methods such as amidite-based solid-phase synthesis can provide nearly quantitative stepwise yields (≥98 % per cycle) and allow for the synthesis of mixed-base, mixed-sugar chimeras in a single run, though the available resin volumes and high solvent usage present scalability challenges. By contrast, enzymatic transcription can provide a gram-to-kilogram per run output in hours in a single-use bioreactor, but can be prone to sensitivity to pyrophosphate build-up and polymerase inhibition. An appreciation of the trade-offs between such methods allows for a rational hybridization of routes, for example using solid-phase synthesis for the preparation of guide strands and enzymatic methods for long mRNA production, enabling high yields while meeting the purity specifications necessary for clinical dose production in a lean single-platform facility, as required by the FDA and EMA to demonstrate consistent chemistry, manufacturing and controls.
Contract development organizations have parallel facilities: explosion proof, low-humidity clean rooms for nucleoside phosphoramidite chemistry and RNAse-free, endotoxin controlled suites for nucleotide IVT biocatalysis. Nucleoside scale-up campaigns are built with tetrazole/phosphonium activator libraries, acid scavenging exhaust and DMT-cation recycling loops. These features are fully documented within ICH Q7 intermediate fabrication guidelines. Nucleotide suites are similarly detailed: WFI loops, single-use mixing bags, pyrophosphate scrubbers and real time RNase assays, cataloged within ICH Q11 drug-substance documentation. Technology-transfer packages differ as a result: nucleoside synthesis pathways are characterized by amidite specifications, coupling cycle parameters and anomeric purity data while nucleotide routes reference enzyme provenance, triphosphate speciation and residual DNA clearance validation. Coordinating the right platform with the capabilities of a CDMO is directly correlated to capital utilization, inspection readiness and speed-to-filing.
We provide a comprehensive range of nucleoside-based raw materials and technical services specifically designed for therapeutic oligonucleotide manufacturing. By focusing on nucleosides as the primary starting materials for chemical synthesis, our solutions support robust solid-phase processes, high sequence fidelity, and scalable manufacturing across RNA and DNA therapeutic platforms.
Our portfolio includes high-purity nucleoside building blocks covering all canonical bases required for RNA and DNA oligonucleotide synthesis. These materials are manufactured under stringent quality standards to ensure tight control of impurities, low moisture content, and consistent batch-to-batch performance. High-purity nucleosides are essential for reliable phosphoramidite preparation and efficient chain elongation. By minimizing contaminating species that can interfere with coupling reactions, our nucleoside building blocks help improve coupling efficiency, overall yield, and final oligonucleotide quality, making them suitable for both development and commercial manufacturing.
To enable precise and efficient oligonucleotide assembly, we offer a wide range of protected and modified nucleosides optimized for conversion into phosphoramidite intermediates. Our materials incorporate well-established base- and sugar-protecting group strategies that are fully compatible with automated solid-phase synthesis. In addition, we supply nucleosides with clinically relevant modifications, including sugar and base modifications designed to enhance stability and biological performance. These protected and modified nucleosides support the synthesis of high-quality phosphoramidites with predictable reactivity and clean deprotection profiles, helping manufacturers maintain control over process complexity and impurity formation.
Choosing the right nucleoside raw materials is a critical decision in oligonucleotide manufacturing. Our technical team provides expert consulting support to help customers select nucleoside building blocks that align with their synthetic strategy, modification requirements, and scale-up plans. We assist with evaluating raw material specifications, assessing compatibility with existing synthesis workflows, and planning transitions from research-grade to GMP-grade materials. This proactive approach helps reduce development risk, avoid late-stage changes, and support regulatory-ready manufacturing processes.
If you need expert guidance on selecting nucleoside-based raw materials for therapeutic oligonucleotide manufacturing, our team is ready to help. Contact us today to discuss your synthesis strategy, request technical documentation or samples, or explore customized solutions to support your development and scale-up goals.
References
Nucleotides contain phosphate groups, while nucleosides do not.
They allow precise control through protecting group and phosphoramidite chemistry.
Mainly in enzymatic synthesis, such as mRNA production via in vitro transcription.
Yes, purity, moisture, and impurity profiles differ significantly.
Yes, incorrect raw material selection can cause yield loss and regulatory issues.

