2'-modified nucleotides are the building blocks of contemporary RNA drugs. The most prevalent 2'-modifications turn labile transcripts into stable, drug-like molecules that are nuclease-resistant, evade innate sensors and high-affinity hybridize. The 2'-hydroxyl is replaced with small alkyl, halo or ether appendages to tune ribose pucker, lower immunogenicity and extend intracellular half-life without loss of base-pair fidelity. The clinical pipeline now features fully 2'-O-methyl, 2'-fluoro, 2'-MOE and mixed 2'-F/Me oligonucleotides, selected to meet the mechanistic need of the therapeutic modality: siRNA, antisense, aptamer or mRNA.
Native RNA is susceptible to degradation by abundant ribonucleases, activates toll-like receptors, and has unfavorable pharmacokinetic properties, and is therefore not well suited for systemic treatment. Addition of a small substituent at the 2'-position enforces a C3'-endo conformation that "locks" the sugar and that concomitantly tightens the target interaction, shields the molecule from pattern-recognition receptors, and sterically blocks the active sites of nucleases. This single atomic modification thus transforms a labile biomolecule into a long-acting drug that can be dosed monthly, or even quarterly.
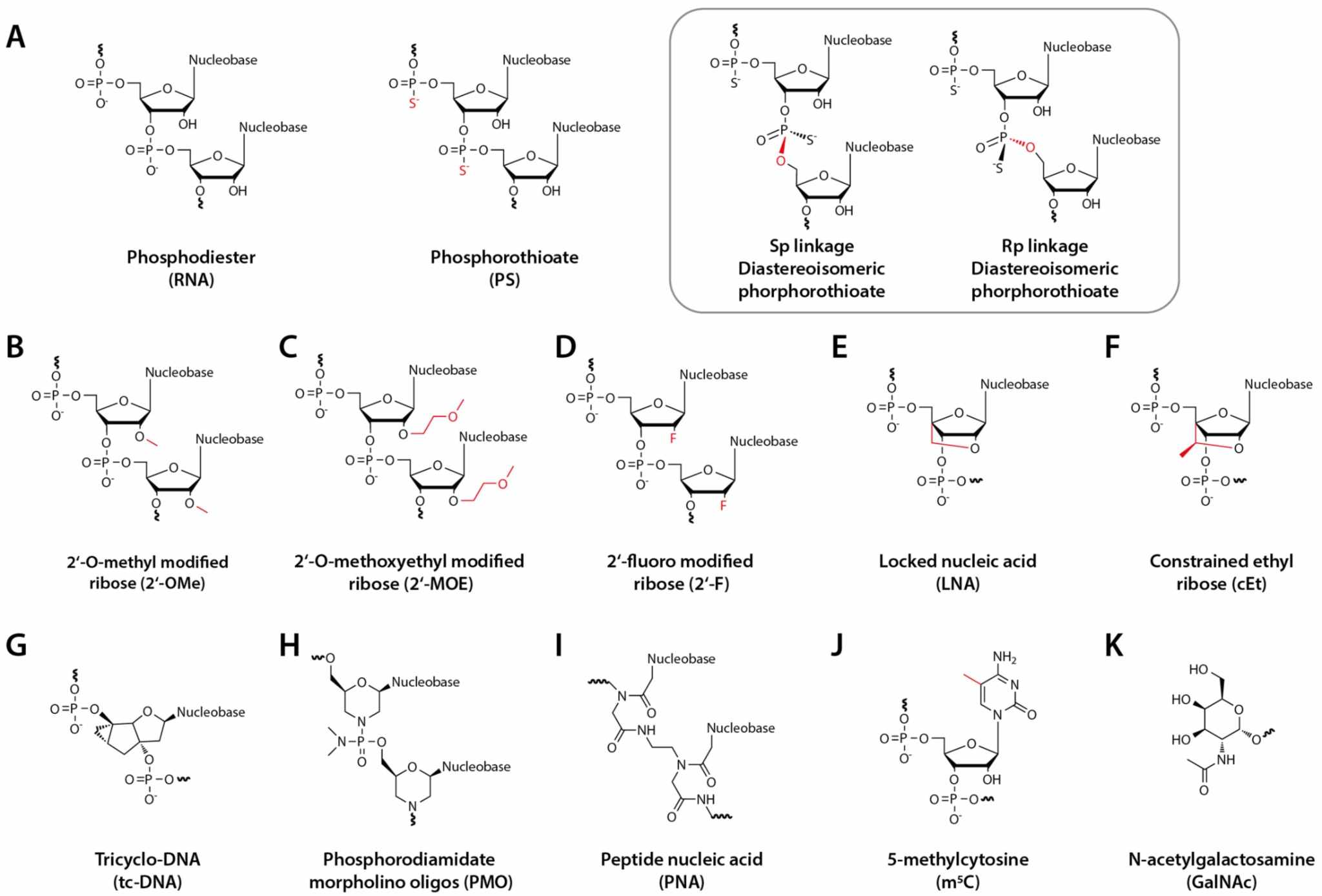 Fig. 1 Structures of AON chemical modifications and the GalNAc conjugate.1,5
Fig. 1 Structures of AON chemical modifications and the GalNAc conjugate.1,5
Native RNA has a reactive 2'-hydroxyl that is an internal nucleophile, causing strand scission at physiological pH and magnesium concentrations. The 2'-hydroxyl is also recognized by RIG-I and TLR7/8, initiating a type-I interferon response that both clears the transcript and causes inflammatory off-target effects. Fast renal filtration and enzymatic degradation limit plasma half-life to minutes, while poor hybridization stability at body temperature reduces on-target potency. The combination of these limitations makes naked RNA unsuitable for much of anything other than transient transfection or local delivery.
2'-O-methyl and 2'-fluoro substituents each extend circulation half-life by several orders of magnitude through a combination of nuclease resistance and reduced renal clearance. The same modifications also raise melting temperature without distorting Watson-Crick geometry, which permits shorter, more specific sequences that minimize off-target hybridization. Critically, 2'-MOE and 2'-F/Me mixed polymers either retain RNase-H recruitment (where desired: gapmer ASOs) or abolish it for steric-block modalities, providing a modular toggle between cleavage-based and occupancy-based mechanisms. The combination of these attributes ultimately translates into sustained gene silencing, improved potency indices and wider therapeutic windows.
The first-in-class systemically active RNA therapeutics, nusinersen, patisiran, inclisiran are characterized by high 2'-MOE and 2'-F content, key to achieving month-long dosing intervals and liver-biased biodistribution. The more recent COVID-19 mRNA vaccines are formulated with N1-methyl-pseudouridine, a 2'-ribose-sparing base modification that still synergizes with 2'-O-methyl residues in the cap-proximal region to enhance translation and reduce innate immunity. For all modalities, the clinical translation of 2'-modified chemistries has not only validated their safety margins, but also paved the regulatory path for next-gen candidates that are now in phase II/III studies.
Table 1 Representative 2'-modifications and their functional impact
| Modification | Stereochemistry | Nuclease Resistance | Immunogenicity | Clinical Use Example |
| 2'-O-methyl | C3'-endo | High | Low | Gapmer flanks, mRNA caps |
| 2'-fluoro | C3'-endo | Very high | Very low | siRNA passenger strand |
| 2'-MOE | C3'-endo | Very high | Very low | ASO wings, siRNA guide |
| 2'-F/Me mixed | Adjustable | Very high | Very low | Next-gen siRNA, aptamers |
The only structural difference between ribose and deoxyribose is the 2'-hydroxyl group, yet this single substituent controls sugar pucker, duplex geometry, backbone flexibility and sensitivity to both enzymatic and chemical cleavage. Under physiological pH and divalent cations the internal nucleophilic hydroxyl group catalyzes strand scission while locking the sugar into a C3'-endo conformation which promotes A-form helices. Consequently, the 2'-position is the primary handle by which medicinal chemists tune RNA stability, binding affinity and immunogenicity without altering the Watson-Crick interface.
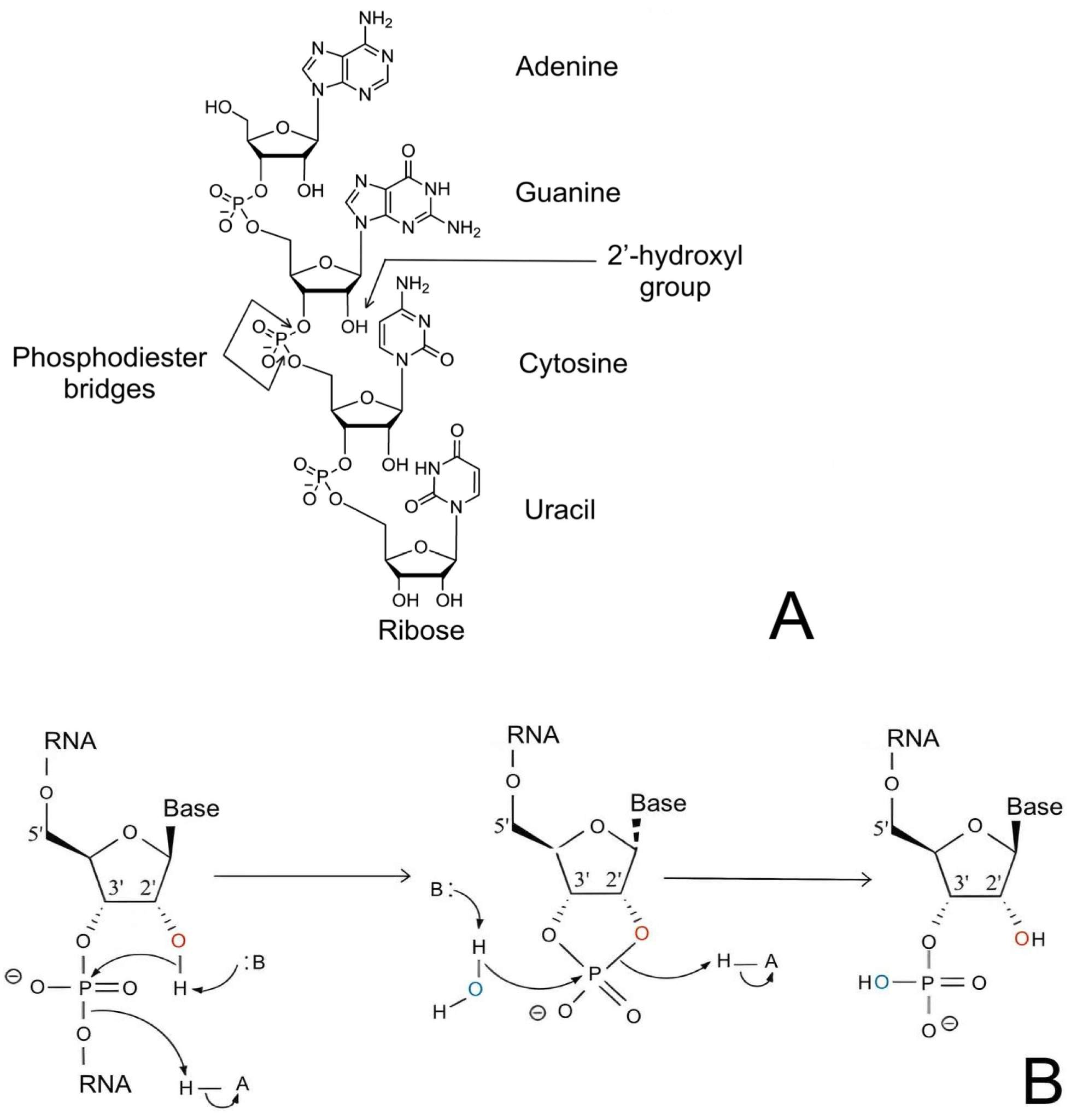 Fig. 2 Structure and hydrolysis of RNA.2,5
Fig. 2 Structure and hydrolysis of RNA.2,5
The 2'-OH biases the furanose ring into a C3'-endo envelope, shortens the phosphate-to-phosphate distance, and broadens the major groove, compared to B-form DNA. This geometry places the hydroxyl within hydrogen-bonding distance of the 3'-phosphate, creating a pre-organised active site for metal-catalysed cleavage that underlies both ribozyme activity and unwanted backbone hydrolysis. In single-stranded regions, the same hydroxyl increases chain rigidity, reducing conformational entropy and thereby raising the thermodynamic cost of duplex unwinding—a property that nature exploits to stabilise tRNA and ribosomal RNA folds.
A-form helices induced by the 2'-OH have deeper major grooves and more extensive base-stacking overlap, which results in higher thermodynamic stability per base pair than equivalent B-form DNA. Conversely, the hydroxyl's nucleophilicity enhances intramolecular transesterification, especially in flexible loops where Mg2+ or Ca2+ can stabilise the pentavalent transition state; this autocleavage pathway is the primary mechanism of chemical degradation for therapeutic RNAs. Substitution at the 2'-position – methylation, fluorination, etherification – removes the nucleophile while maintaining the C3'-endo pucker, trading innate lability for drug-like durability without loss of helical geometry.
Deoxyribose was chosen by evolution to store the genome precisely because the lack of the 2'-OH removes the prevailing hydrolytic sink; DNA phosphodiester bonds are orders of magnitude more stable at physiological ionic strength and pH. The C2'-endo sugar pucker favored by deoxyribose also results in a wider, shallower major groove, which can host regulatory proteins while remaining chemically inert. 2'-Modifications would confer no stability advantage to DNA, and would distort the B-form helix, which explains why nature reserves such embellishments for the more transient, regulatory world of RNA.
The 2'-position is the foremost handle available to the medicinal chemist for modulating RNA properties: small alkyl or halide substituents at this position transform the reactive 2'-hydroxyl group into a pre-organized, lipophilic, and nuclease-resistant "stub." Such groups also result in a more rigid duplex geometry and dampening of Toll-like receptor recognition. The three most commonly used 2'-modifications in clinical development today are 2'-O-methyl, 2'-fluoro, and 2'-O-methoxyethyl (compare figure at right), and others such as 2'-O-N-methyl-acetamido or 2'-cyanoethyl are being explored to further push the affinity/stability frontier. Each modification is chosen according to positional need (guide vs passenger, gapmer wing vs core), delivery route and immunogenicity tolerance.
Substitution of the 2'-OH with a methyl ether locks the sugar into a C3'-endo pucker that increases Tm and removes the internal nucleophile required for backbone hydrolysis. The group is small enough that it can fit into natural active sites so 2'-OMe strands can recruit RNase-H when included in gapmer cores but can resist degradation when used as steric blocking groups in splice-switching oligos. Because the modification is naturally found in tRNA and rRNA, it is well tolerated by human polymerases and causes little innate immune activation, making it the default modification of choice for mRNA caps and siRNA passenger strands.
The high electronegativity of fluorine abstracts electron density from the ring oxygen, favouring a sugar bias towards the C3'-endo conformation and increased hydrogen-bond fidelity without any additional steric encumbrance. The C–F bond is chemically unreactive so that 2'-F nucleotides are nuclease blind and the A-form geometry required for RISC loading is maintained. In the clinic, 2'-F is typically alternated with 2'-OMe in siRNA guide strands to introduce a thermodynamic asymmetry that biases proper strand selection, and the modification has been dosed at high cumulative levels in hepatic targeting programs.
Adding another methylene group to the 2'-O-alkyl chain as a flexible ethylene-glycol unit increases steric bulk and hydrophilicity to further protect the phosphate backbone while retaining the C3'-endo pucker. As MOE increases affinity more than methyl, it allows for shorter antisense sequence, thereby reducing off-target hybridisation and lowering the cost of manufacturing. The additional ether oxygen also provides a hydrogen-bond acceptor and further increases aqueous solubility, an attractive property for sub-cutaneous depot formulations. Gapmer antisense oligos with MOE wings show month-long tissue half-lives and have reached phase III without any indication of cumulative toxicity.
Second generation motifs aim to integrate the desirable traits of methyl, fluoro and MOE, while providing new pharmacological handles. 2'-O-N-methyl-acetamido adds a polar carbonyl that improves cellular uptake through amino-acid transporters; 2'-cyanoethyl presents a metabolically soft chain that can be cleaved in endosomes to regenerate the natural RNA for transient activity; locked and unlocked nucleic acids lock or unlock the sugar pucker for extreme affinity or RNase-H compatibility. These analogues are under investigation in allele-selective siRNAs and self-amplifying mRNA templates to redefine potency, duration and tissue selectivity.
Table 2 Comparative profile of clinical-stage 2'-modifications
| Modification | Steric Size | Hydrophilicity | Affinity Gain | Immunogenicity | Typical Use |
| 2'-OMe | Small | Low | Moderate | Very low | mRNA cap, passenger |
| 2'-F | Very small | Very low | High | Very low | Guide strand hotspots |
| 2'-MOE | Medium | High | High | Low | ASO wings |
| 2'-NHAc | Medium | High | Moderate | Low | Uptake-enhanced siRNA |
| 2'-cyanoethyl | Medium | Medium | Moderate | Low | Cleavable pro-RNA |
Modification of the native 2'-hydroxyl to small alkyl, halo or ether groups can transform the ribose from a reactive scaffold into a stable, drug-like framework. The substitution removes the intramolecular nucleophile which facilitates backbone cleavage, enforces a C3'-endo pucker which tightens duplex packing and sterically shields the phosphodiester from enzyme active sites. These orthogonal benefits are additive over the course of dozens of coupling cycles, and can lead to multi-log improvements in circulating half-life with minimal loss of base-pair fidelity or translational efficiency.
The 2'-OH is the Achilles heel of RNA: it is an internal nucleophile that attacks the adjacent phosphate, which promotes strand scission under physiological magnesium and pH. Modifying the hydroxyl with a methyl, fluoro or methoxyethyl group disrupts the sugar backbone's connection to the cleavage pathway and blocks exonuclease active sites from accessing the modified face sterically. 2'-O-methyl and 2'-MOE oligos show markedly slower digestion by serum RNases, and even a sparse sprinkling of 2'-fluoro residues at the 3'-terminus is sufficient to inhibit 3'-exonucleases that would otherwise "nibble" the strand within minutes of systemic exposure.
2'-modifications push the sugar into the C3'-endo conformation that is natural for A-form helices. This increases the base-stacking overlap and thereby also melting temperature. Molecular-dynamic studies have shown that the reduced flexibility of the rise parameter (not only sugar pucker) is in strong correlation with duplex stability: 2'-O-cyanoethyl and 2'-MOE strands display lower deformability along the helical axis, which results in tighter and more predictable Tm profiles. This higher duplex stability enables the use of shorter and more specific sequences, reducing off-target hybridization and manufacturing costs while retaining activity under physiological salt concentrations.
RNA undergoes both enzymatic attack and vulnerability to base-catalyzed hydrolysis and oxidative damage prompted by metals. 2'-fluoro and 2'-MOE substitutions preclude the proton required for β-elimination pathways, suppressing strand scission under mild alkaline conditions typically employed during deprotection or formulation. These chemical substitutions reduce redox sensitivity at the neighboring phosphate through decreased local electron density which results in reduced mutagenic 8-oxo-guanine adduct formation during extended storage or freeze-drying processes. Collectively, these chemical defences extend shelf-life from days to years and permit depot formulations that release intact oligonucleotide over monthly dosing intervals.
2'-modifications work as molecular rheostats, that concomitantly 'clamp down' binding to the on-target RNA and 'loosen' off-target interactions with partially mismatched transcripts. Steric locking of the sugar into the C3'-endo conformation and a change in steric/electronic environment of the minor groove, these minor substituents increase the thermodynamic discrimination between perfectly matched and off-perfect duplexes, resulting in increased gene silencing efficacy at lower concentrations with minimal collateral gene regulation.
Enforced C3'-endo sugar pucker by 2'-O-methyl, 2'-fluoro or 2'-MOE modifications reduce the rise between phosphates and increase the extent of base-stacking overlap. This can increase melting temperature without increasing sequence length, as the enthalpy gain from the shorter oligo can achieve similar affinity to longer native counterpart. The energetic benefit can be important where the therapeutic window is ultimately limited by hepatic clearance, for example. Fluorine's electronegativity additionally hardens the sugar ring, lessening the entropic penalty upon hybridisation and permitting sub-nanomolar occupancy of structured viral and/or oncogenic RNAs that might otherwise be recalcitrant to binding.
Off-target silencing is largely mediated by seed-region (positions 2–8) complementarity to off-target mRNAs. Placement of 2'-O-methyl at position 2 of the guide strand destabilises these weak duplexes while maintaining high affinity to the perfectly matched target, reducing the number of mis-regulated genes by about half. Extending the pattern—alternating 2'-OMe/2'-F or with short LNA inserts—shifts the thermodynamic balance even further, so that only transcripts with long stretches of complementarity are cleaved. Because the modification is steric rather than sequence-specific, the same strategy can be transferred between targets without redesigning the entire chemical scaffold.
2'-substituents can further stabilise RNA duplexes by biasing equilibrium towards A-form helices and discouraging non-canonical folds (bulges, G-quadruplexes, etc.) which sequester the guide strand and limit RISC loading. In self-folding transcripts (aptamers, ribozymes), 2'-MOE or 2'-F residues at strategic positions rigidify stems while maintaining flexible loops, augmenting target recognition and/or catalytic turnover. Overly aggressive modification can over-stabilize hairpins, which in extreme cases traps the strand in a stem with limited ability to separate and reduces potency. Design algorithms now include free-energy penalties for each 2'-chemistry to determine the optimal pattern to maximize on-target affinity without trapping the oligo in inactive conformations.
In the context of siRNA, antisense and mRNA, 2'-modifications function as modality-specific dials to tune nuclease resistance, protein binding, and immunogenicity, without affecting Watson-Crick fidelity. The same methyl, fluoro or MOE substituent can have radically different purposes – silencing RISC loading in the context of siRNA, recruiting RNase-H in gapmers, or masking cap-adjacent nucleotides in mRNA, for example – depending on which elements are in its immediate neighbourhood, and which sets of enzymes must be engaged or evaded in each case. Successful clinical translation of these modalities therefore demands a modality-aware design grammar that tightly aligns modification pattern with delivery route, mechanism of action and duration of effect.
Asymmetric loading of 2'-modifications in siRNA is another approach. Guide strand chemistries are 2'-fluoro or 2'-O-methyl positions 1–8 to increase seed affinity without decorating the cleavage site (position 10–11) with bulky substituents that could interfere with Argonaute cleavage, while the passenger strand is more heavily clad with 2'OMe- or 2'MOE to avoid off-target loading and to reduce TLR7/8 activation. This "stability-asymmetry" strategy provides plasma half-lives in days rather than minutes, supports sub-cutaneous GalNAc delivery and has led to marketed drugs that silence hepatic transcripts for months after single dosing.
Gapmer antisense oligos have 2'-MOE or 2'-OMe "wings" flanking a DNA "core": the modifications increase affinity and nuclease resistance, and the unmodified DNA recruits RNase-H to cleave the target. 2'-MOE is used for wings because the ethylene-glycol ether improves aqueous solubility and tissue half-life, reducing dosing frequency; 2'-fluoro is avoided in wings because its small size over-stabilises DNA-RNA hybrids, causing non-specific RNase-H cleavage. The chemistry has yielded FDA and EMA-approved drugs that lower hepatic mRNA levels for months after sub-cutaneous injection.
In mRNA, 2'-modifications are used sparingly but strategically: 2'-O-methyl is embedded in the first 3–4 nucleotides of the 5'-cap adjacent to N1-methyl-pseudouridine to suppress RIG-I recognition while maintaining translational efficiency; internal 2'-OMe residues are sometimes added into U-rich tracts to eliminate residual TLR8 stimulation without changing codon usage. Since the mRNA must be translated, 2'-substitution is used more sparingly than in siRNA or RISC where it can be more extensive; usually <5 % of all nucleotides are substituted to avoid ribosomal stalling and loss of protein production.
siRNA is more tolerant of heavy 2'-coverage as RISC can still cleave the guide strand; antisense requires a DNA "gap" for RNase-H; mRNA needs a cap-proximal 2'-OMe veil but an otherwise native backbone for translation. Delivery route further constrains choice: GalNAc-siRNA favours 2'-F/Me blends for high-affinity hepatocyte uptake, while lipid-nanoparticle mRNA prefers minimal 2'-alteration to avoid particle destabilisation. Early alignment of modification pattern with modality-specific enzymology and delivery physics prevents late-stage bridging studies and accelerates regulatory approval.
Table 3 Modality-specific deployment of 2'-chemistry
| Modality | Typical Pattern | Enzyme Engaged | Delivery Route | Key Risk if Over-Modified |
| siRNA | Asymmetric F/Me | RISC | GalNAc, LNP | Loss of guide-strand cleavage |
| Antisense gapmer | MOE wings + DNA core | RNase-H | Sub-cutaneous | Non-specific RNase-H activation |
| mRNA | Cap-proximal Me only | Ribosome | LNP, ionisable | Translational stalling |
The industrial manufacture of 2'-modified nucleotides is defined by the challenge to introduce a single stereogenic substituent into a heavily functionalised ribose in the context of an orthogonal protection on base, phosphate and 5'-hydroxyl. The additional alkylation or fluorination step extends the synthetic tree, increases the solvent volume requirement and adds new classes of impurities (diastereomeric phosphoramidites, trace fluoride or metal catalyst) that must be eliminated prior to the monomer release under cGMP. Success is therefore critically dependent on an early convergence of route design, protecting-group stability and analytical fate mapping so that the final kilogram-scale batch recapitulates the impurity envelope of the gram-scale toxicology lot.
Incorporating a 2'-O-methyl, 2'-fluoro or 2'-MOE group regioselectively so as to leave the 3'-position available for phosphitylation is difficult. Classical methods based on transient 2',3'-orthoester formation followed by partial opening require anhydrous conditions and cryogenic control to prevent 3'-OH over-alkylation. Fluorination routes use DAST or XtalFluor reagents that release HF in situ: even at the ppm level, water hydrolyses the reagent and generates 2',3'-difluoro byproducts that co-crystallise with the desired monomer and are chromatographically silent. Yield losses are cumulative: a 5% per step loss that is negligible at 50 g levels becomes a multi-kilogram shortfall for 100 kg of starting sugar, with the plant being overrun by necessary campaign extensions and oligonucleotide production being delayed.
Installation of the 2'-modification precedes global base and phosphate protection as reagents used for sugar transformation (strong base, fluoride ion, metal catalyst) would cleave conventional benzoyl or cyanoethyl masks. A common sequence thus introduces the 2'-substituent on a partially protected nucleoside, followed by transient silylation of the 3'-OH to prevent competing phosphorylation, and finally installs base amides and 5'-DMT under mild conditions that do not epimerise the newly created stereocentre. Failure to maintain this choreography results in protecting-group migration (e.g. benzoyl sliding from base to sugar amine) or silyl group scrambling that generates diastereomeric phosphoramidites impossible to separate by crystallisation alone. Route scouting therefore includes forced-spiking studies where each protecting arm is subjected to the harshest conditions of the subsequent step, ensuring that no new impurity above the genotoxic qualification threshold is created during scale-up.
The final monomer must be demonstrated to contain a single 2'-epimer and be free of regio-isomeric contaminants, as well as residual metal or fluoride, both of which will poison downstream phosphitylation. Orthogonal HPLC methods (ion-pair and hydrophilic interaction) are method validated for specificity against a library of authentic side-products; IC is used to quantify fluoride at ppb levels, and ICP-MS is used to map catalyst metals introduced during either fluorination or etherification. Because 2'-modified nucleosides are often crystallized as mixed solvates, a qualified drying curve links residual solvent to protecting-group integrity, and this validation is also important for prevention of hydrate formation, which would increase the moisture content above the critical limit once the amidite is re-dissolved in anhydrous acetonitrile. Finally, a stability-indicating method subjects the monomer to forced degradation (acid, base, oxidation) to confirm that any new peak can be resolved from the main component and that future cGMP lots will be rejected before they reach the oligonucleotide synthesis suite.
We offer a comprehensive portfolio of 2'-modified nucleotide building blocks specifically designed for RNA therapeutic development. By combining high-quality modified monomers with deep technical expertise in oligonucleotide chemistry, we support the development of RNA drugs with enhanced stability, potency, and safety across discovery, development, and commercial manufacturing.
Our product portfolio includes a full range of 2'-O-methyl (2'-OMe), 2'-fluoro (2'-F), and 2'-O-methoxyethyl (2'-MOE) nucleotides, covering all canonical RNA bases. These modifications are widely used in siRNA, antisense oligonucleotides, and other RNA therapeutic modalities to improve nuclease resistance, binding affinity, and pharmacokinetic profiles. All 2'-modified nucleotides are manufactured to high purity standards with tightly controlled impurity and moisture profiles, ensuring consistent performance during oligonucleotide synthesis and downstream processing. Our broad modification portfolio allows customers to design optimized RNA sequences tailored to specific therapeutic and delivery requirements.
To enable efficient chemical oligonucleotide synthesis, we supply protected 2'-modified nucleoside monomers that are fully compatible with phosphoramidite chemistry. These monomers incorporate well-established, orthogonal protecting group strategies that support high coupling efficiency, controlled reactivity, and clean deprotection. Our protected 2'-modified monomers are designed to integrate seamlessly into automated solid-phase synthesis workflows, helping manufacturers maintain sequence fidelity, high yields, and reproducible performance, even in highly modified RNA constructs.
For programs requiring non-standard modifications or tailored design strategies, we offer custom modification and scale-up support. Our technical team assists with modification selection, synthetic route development, protecting group optimization, and impurity control to ensure that custom 2'-modified nucleotides are chemically robust and scalable. By considering manufacturability early in development, we help customers reduce technical risk and ensure that modified nucleotides remain suitable for long-term supply as programs advance toward clinical and commercial stages.
We provide GMP-ready supply options for 2'-modified nucleotides to support clinical and commercial RNA therapeutic manufacturing. Our materials are supported by clear specifications, certificates of analysis, traceability, and change control processes, helping customers meet regulatory and quality expectations. This regulatory-aware supply approach enables smooth transitions from development to GMP production, minimizing the risk of late-stage raw material changes and supporting long-term manufacturing continuity.
If you are developing RNA therapeutics that require high-quality 2'-modified nucleotides, our team is ready to support your program. Contact us today to request technical information, samples, or quotations, or to discuss custom modification strategies and GMP-ready supply solutions tailored to your RNA development needs.
References
Nucleotides modified at the 2' position of the ribose sugar.
It strongly affects RNA stability and conformation.
2'-O-Me, 2'-F, and 2'-MOE.
Yes, many approved RNA drugs use them.
Yes, they require specialized chemistry and protection.

