2'-O-methyl (2'-OMe) nucleotides are the work-horse of clinical RNA therapeutics, because a single methyl ether simultaneously removes the 2'-hydroxyl nucleophile, raises duplex melting temperature and silences toll-like receptors. Present in every approved siRNA and in the wings of marketed antisense oligos, the modification delivers month-long tissue half-lives and sub-nanomolar potencies without increasing synthetic complexity, making it the first-choice sugar edit when speed-to-clinic and cost-of-goods matter.
Native RNA is vulnerable to serum ribonucleases within minutes, and elicits strong innate immune signalling; 2'-OMe replaces the chemically reactive hydroxyl with a metabolically silent methyl group that occludes both of the above degradation pathways, while also biasing the sugar toward a C3'-endo pucker that is favourable for high-affinity base pairing. As the modification is naturally present in tRNA and rRNA, human polymerases are permissive of it, which lets developers include 2'-OMe in RNAs at strategic positions without having to redesign synthesis protocols or worry about idiosyncratic toxicity.
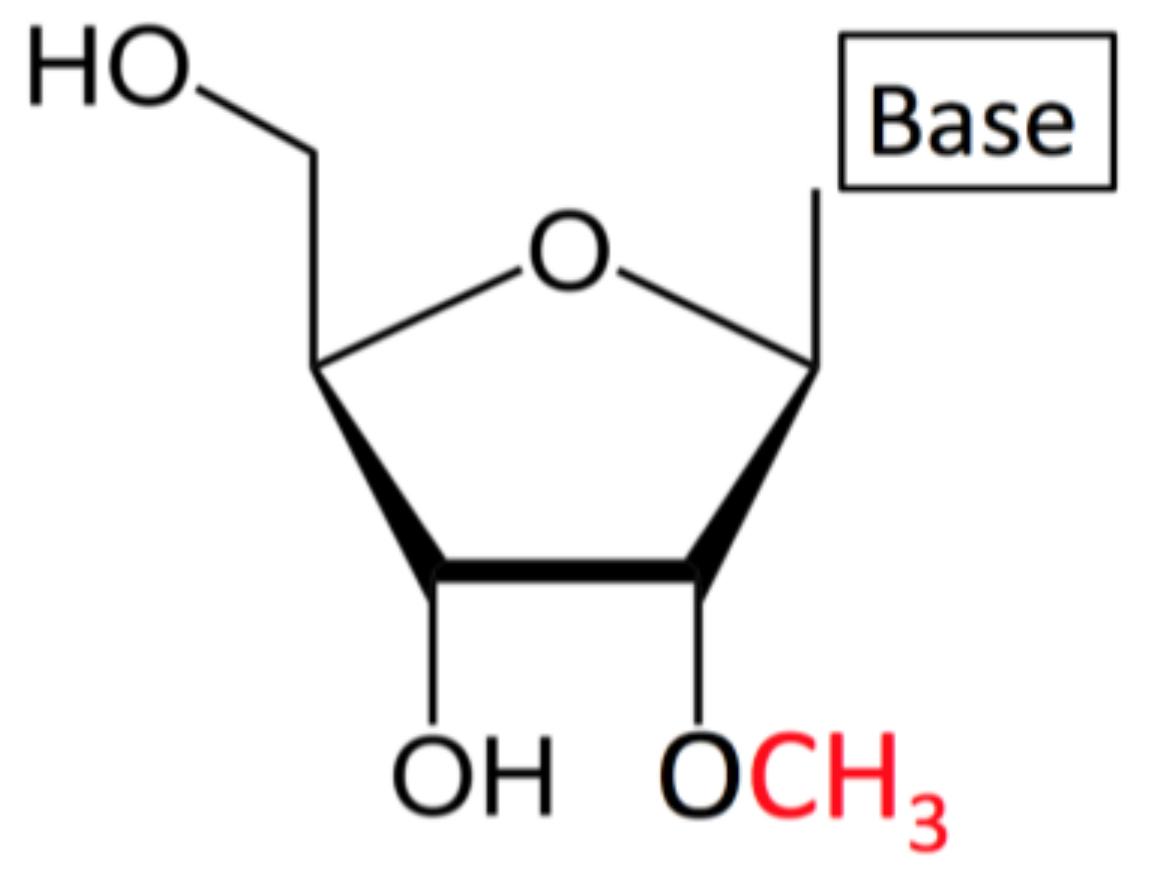 Fig. 1 2'-O-methylated ribonucleoside (Nm): A –CH3 group is replacing the –H at the position 2' in the ribose moiety.1,5
Fig. 1 2'-O-methylated ribonucleoside (Nm): A –CH3 group is replacing the –H at the position 2' in the ribose moiety.1,5
The drawbacks of unmodified RNA include rapid self-catalyzed hydrolysis by the 2'-hydroxyl, clearance within minutes through renal filtration, and activation of TLR7/8 and RIG-I which trigger inflammatory cytokines. These limitations require sophisticated delivery vehicles or extensive chemical modification; 2'-OMe overcomes all three liabilities in one, inexpensive step and allows for sub-cutaneous delivery and a monthly dosing regimen without loss of gene-silencing efficacy.
Early structure–activity relationships showed that 2'-OMe increased melting temperature without sacrificing recruitment of RNase-H when placed in the wings of gapmers. This combination of features ported into increased tissue half-life in rodent and non-human primate studies. The modification rapidly replaced previous 2'-F or phosphorothioate-only designs, as it provides similar stability with reduced immunogenicity and simpler synthetic supply chain, and has become the go-to sugar modification for siRNA, antisense and mRNA cap applications.
All FDA-approved siRNAs to date have 2'-OMe in the passenger strand, and many also in the seed region of the guide strand to increase stability and reduce off-target noise, while the antisense drugs such as nusinersen utilize full 2'-OMe wings on either side of a central DNA gap to provide month-long CNS exposure after intrathecal injection. The compatibility of the modification with standard phosphoramidite chemistry and its low cost of goods have made 2'-OMe the backbone of first-in-class therapies for hereditary transthyretin amyloidosis, spinal muscular atrophy and hypercholesterolaemia, validating 2'-OMe as a commercially viable platform.
Table 1 Clinical utilisation pattern of 2'-OMe in approved RNA drugs
| Drug Modality | 2'-OMe Location | Clinical Benefit | Regulatory Status |
| siRNA passenger | Full strand | Stability, low Immuno | Multiple approvals |
| siRNA guide seed | Positions 1–8 | Off-target reduction | Standard of care |
| ASO wings | Full 2'-OMe | Month-long half-life | CNS and liver indications |
| mRNA cap | First 3 nucleotides | TLR silencing | Vaccines & therapeutics |
The 2'-O-methyl nucleotide is characterized by a single ether oxygen linking C-2' of the ribose to a methyl group, a substitution that replaces the native hydroxyl with a non-hydrogen-bond-donating lipophilic cap. The subtle edit simultaneously abolishes the intramolecular nucleophile normally responsible for triggering backbone cleavage, while the methyl steric bulk pre-organises the sugar into a more rigid North-type pucker without introducing a new stereocentre or perturbing the Watson–Crick edge of the base.
The modification is introduced by methylation of the 2'-alkoxide with methyl iodide or methyl triflate under strictly anhydrous conditions, generally after temporary protection of the 3'- and 5'-hydroxyls as acetals or silyl ethers to prevent formation of regio-isomers. The resulting methyl ether is stable to both the acid detritylation pulses used in solid-phase elongation as well as the final aqueous ammonia treatment and so it remains intact throughout the entire synthetic effort. As no new chiral center is created, the phosphoramidite synthesized from the modified nucleoside behaves in exactly the same way as the native nucleoside in coupling kinetics, and can be substituted into an existing synthesis scheme without the need to re-optimize cycle times or activator concentrations.
Crystallographic and NMR data show that the 2'-O-methyl group pre-organizes the furanose into the C3'-endo (North) pucker by sterically disfavoring the C2'-endo (South) envelope. This reduces the axial rise between adjacent bases and increases the helical twist, resulting in a more compact A-form duplex. The restricted geometry also minimizes the entropic cost of target binding, so each modified residue cumulatively increases the melting temperature without requiring additional GC content. Critically, the North pucker is consistent with the catalytic geometry of RNase H and RISC, so strands with dispersed 2'-O-methyl substitutions can direct cleavage or translational repression depending on the local context.
Compared to unmodified RNA, the 2'-O-methyl analogue lacks the hydrogen-bond donor that properly orients a water for inline attack of the 3'-phosphate, so serum, endosomal and cytosolic hydrolytic half-lives are much longer. The methyl group also sterically shields the phosphate from ribonuclease active-site binding, further prolonging its metabolic stability. Electrophoretic mobility changes slightly towards the anode due to the ether oxygen being less polarisable than the parent hydroxyl, a feature that was taken advantage of during the development of analytical methods to separate modified and unmodified strands. The lack of a labile 2'-proton also eliminates the pH dependent β-elimination reaction that otherwise leads to RNA fragmentation under mild basic storage conditions, providing a greater pH stability window and longer shelf-life for the formulated drug product.
Replacement of the 2'-hydroxyl by a methyl ether both deprives the nucleic acid of the intramolecular nucleophile that mediates backbone cleavage, and constrains the sugar into a C3'-endo pucker that results in more compact duplex packing. The alteration is small enough to leave the Watson-Crick edges unperturbed but bulky enough to sterically protect the nucleic acid against both endo- and exo-nucleases, resulting in multi-log increases in serum half-life without loss of hybridization fidelity or translational efficiency.
The 2'-OMe group protrudes into the minor groove and sterically shields the scissile phosphodiester from RNase A and serum endonucleases. Since the methyl ether abolishes the 2'-OH needed for internal transesterification, this modification also prevents the predominant chemical cleavage pathway under physiological magnesium and pH, producing oligonucleotides that can survive for days in biological media rather than minutes for native RNA.
Uniform 2'-OMe incorporation biases the strand towards a homogeneous C3'-endo sugar pucker along the entire length, leading to enhanced base-stacking overlap and increased melting temperature. Entropic gain is greatest in RNA: RNA duplexes, allowing for shorter, more sequence-specific strands that can maintain high affinity while diminishing off-target hybridisation (property which has been exploited in both siRNA guide strands and antisense wings).
In addition to this enzymatic deactivation, 2'-OMe also inhibits base-catalyzed hydrolysis and metal-mediated oxidative damage by eliminating the β-elimination promoting proton and reducing the local electronic density at the phosphate. As a result of this modification, shelf-life is increased from hours to years under ambient storage conditions, and depot formulations become possible that release intact oligonucleotide over monthly dosing intervals without the formation of mutagenic 8-oxo-guanine or abasic sites.
2'-O-methyl nucleotides are the most common chemical modification that make a labile, immunogenic siRNA duplex into a drug like molecule with month-long systemic activity. Replacement of the native 2'-hydroxyl with a metabolically silent methyl ether modification concurrently blocks nuclease attack, elevates melting temperature and silences toll-like receptors, enabling sub-cutaneous GalNAc conjugates to achieve hepatic gene silencing with single milligram doses. The same small edit now incorporated—position by position—into every clinically approved siRNA, has validated 2'-OMe as the gold-standard sugar chemistry for RNAi therapeutics.
The choice of asymmetric chemistry is founded on thermodynamic partitioning. Making the passenger strand "invisible" to the RNA-interference (RNAi) machinery (by full 2'-OMe coverage) prevents it from competing for Argonaute loading, thereby focusing all of the silencing capacity on the guide strand. A full 2'-OMe passenger also removes residual TLR7/8 agonism, which is important for safe re-administration of the duplex. Methylation on the guide is strategically limited: the seed (nucleotides 2–8) is maximally decorated for higher affinity and specificity while the cleavage centre (nucleotides 10–11) is mostly native or lightly fluorinated to allow the necessary conformational flexibility for endonucleolytic scission. This regionalized pattern is supported by deep-sequencing data demonstrating that the passenger-strand-derived off-target signal is reduced by more than an order of magnitude when the guide strand is asymmetrically 2'-modified, with the on-target knock-down window being largely retained by the parent unmodified sequence. The 3' overhangs of both strands are also fully methylated to sterically block 3'-exonucleases, thereby increasing half-life from minutes to days and allowing for once-monthly sub-cutaneous doses that have become the industry standard for GalNAc-conjugated siRNA drugs.
Off-target silencing is mainly attributed to partial complementarity within the seed region of the guide strand. Strategic placement of 2'-OMe residues in the seed sequence increases the enthalpic penalty for bulged or mismatched hybrids, shifting RISC to a preference for perfectly complementary targets. Genome-wide microarray analysis shows that a single 2'-OMe edit to position 7 of the guide strand can eliminate over 50% of seed-dependent off-target signatures without loss of on-target cleavage efficiency. In addition, the methyl ether occludes the minor-groove face recognized by TLR7/8, preventing the cytokine burst (IFN-α, TNF-α) that typically accompanies high-dose unmodified siRNA. This dual advantage of thermodynamic discrimination and immune quiescence has made 2'-OMe the default edit for clinical candidates, enabling developers to comfortably exceed the very strict off-target thresholds established by the regulatory agencies while maintaining the high gene-silencing potency needed for therapeutic efficacy.
The entropic gain from the C3'-endo locked puckering is that the guide strand is already pre-shaped to allow for the most favorable base-pair geometry when it reaches the RISC loading complex. This means that the kinetic barrier for strand transfer is lower and the activation of RISC is faster, which in turn increases the proportion of guide strands that participate in cleavage. It has also been observed that 2'-OMe-rich siRNAs reach maximal knock-down of the target in the liver within 24 hours, whereas multiple doses over the course of a few days are required to achieve similar levels of mRNA reduction with unmodified duplexes. The increased stability of the RISC complex also increases the half-life of the guide strand within RISC from hours to days, which results in longer-term gene silencing that can last weeks following a single sub-cutaneous injection. Finally, the increased thermodynamic stability allows shorter sequences (19–21 mer) with the same high affinity to be used, which reduces manufacturing cost and minimizes the potential for non-specific hybridization to partially complementary transcripts.
Contemporary pipelines use ML models to consider the thermodynamic contribution of each possible 2'-OMe substitution in the context of its expected effect on RISC loading, seed match off-target stability, and elevation of liver enzymes (transaminases). The general pattern that has emerged from these datasets places 2'-OMe on every nucleotide of the passenger strand (disabling it) and uses a punctate distribution on the guide strand: densely packed in the seed for increased specificity, light in the cleavage centre for catalytic proficiency, and densely packed in the 3' overhang to protect from exonuclease degradation. Over-methylation (>~70 % of the guide strand) can over-stabilise the duplex, which leads to poor strand discrimination and saturation of the RISC machinery, which in primates is reflected by elevation of liver enzymes. Under-methylation leaves a degree of immunogenicity and shortens plasma half-life. Thus, the ideal balance is refined iteratively through RISC-loading assays in-vitro, off-target transcriptome analysis, and dose-ranging toxicology studies to produce a modification pattern which gives the maximal on-target potency at the lowest effective dose with a safety profile suitable for monthly or even quarterly dosing.
2'-O-methyl nucleotides convert traditional antisense oligonucleotides (ASOs) into biopharmaceuticals that possess high affinity for their targets while also displaying an extended half-life in tissues with low inflammatory risk. Incorporated as "wings" around a DNA or phosphorothioate "gap", the methyl ether "waist" cinches duplex geometry, precludes exonuclease activity and masks the minor-groove face that would otherwise activate innate immune sensors. The net result is a gapmer structure that harnesses RNase-H for catalytic destruction of target RNAs while the 2'-OMe domains provide pharmacokinetic and safety "buffering" that supports monthly or quarterly dosing schedules in the clinic.
Uniform 2'-OMe incorporation biases the sugar pucker toward C3'-endo, increasing base-stacking overlap and hence melting temperature without increasing sequence length. The enthalpic bonus allows shorter ASOs (16–18 nt) to retain high-affinity binding to structured pre-mRNA or GC-rich regions that would otherwise resist hybridization. Because the modification is steric rather than electronic, it discriminates more effectively against single-base mismatches than phosphorothioate linkages alone, sharpening the therapeutic index and reducing the need for ultra-high doses that might saturate tissue uptake pathways.
One of the more mature applications of ASOs is in exon skipping strategies. The ASO needs to be strong enough to bind to a site that can compete off endogenous spliceosomal proteins while also being stable enough to persist for weeks in the nucleus to allow proper splicing. 2'-OMe modifications provide this stability while not disturbing the register necessary to select the appropriate splice site. The presence of the methyl ether sterically blocks the binding of hnRNPs that would otherwise promote exon inclusion, effectively biasing the splice-code to preclude the inclusion of disease-causing exons. Absence of an enzymatic target al.o lengthens the time window for efficacy as the ASO need not be continually bound to its target but rather remain bound until the transcriptional half-life dictates the target pre-mRNA is no longer available. A number of recent clinical programs have been designed with this strategy to shift splicing in diseases such as Duchenne muscular dystrophy, spinal muscular atrophy, and inherited blindness, where the shift in reading frame requires a single base precision to preclude the introduction of new cryptic splice sites.
The incorporation of 2'-OMe moderately increases lipophilicity, which results in increased plasma protein association and slower renal filtration, prolonging its circulation from minutes to hours. Following tissue distribution, the modification confers resistance to extracellular RNases, which in turn promotes ASO diffusion in interstitial spaces and cellular entry through endocytosis, which is less hindered by 2'-OMe than highly PS-modified ASOs. Within the cell, 2'-OMe-containing segments are found to partition into both the cytosol and the nucleus, which is compatible with direct cytoplasmic mRNA knock-down as well as nuclear pre-mRNA splicing regulation without additional carrier systems.
Incorporation of 2'-O-methyl (2'-OMe) nucleotides is a well-established strategy to improve the immunogenicity and safety profile of siRNA and antisense oligonucleotide therapeutics. The 2'-OMe modification reduces recognition by innate immune sensors such as Toll-like receptors (TLRs) and other RNA-sensing pathways, thereby minimizing unwanted immune activation. At the same time, 2'-OMe nucleotides maintain favorable hybridization properties, allowing potent target binding without compromising biological activity. This balance between reduced immune stimulation and preserved efficacy makes 2'-O-methyl modification a preferred choice in the design of clinically successful RNA-based drugs.
The 2'-OMe sugar masks the minor-groove pattern recognized by TLR7/8 and RIG-I, removing the interferon-α and TNF-α bursts that accompany administration of high doses of unmodified oligonucleotides. This "immune silence" is preserved even when the ASO is given at gram-scale cumulative exposure, thus chronic treatment of slowly progressive genetic diseases can be achieved without the flu-like symptoms or hepatic transaminase spikes that historically limited dose escalation. The modification therefore converts a potentially pro-inflammatory molecule into a stealth therapeutic that can be up-titrated to maximal pharmacological effect.
Repeated chronic intrathecal or sub-cutaneous administration of 2'-OMe-rich ASOs has been linked to less injection-site reactions, lower complement activation and minimal renal accumulation as compared with first-generation phosphorothioate-only molecules. The methyl ether's neutral charge lessens non-specific binding to coagulation factors and platelet proteins, which has translated into cleaner safety panels and enabled higher cumulative doses for neuro-muscular or ocular indications where long-term exposure is unavoidable.
Finding the balance between these opposing forces required extensive back and forth in-vitro/in vivo correlations between the degree of 2'-OMe modification with PD potency and toxicity outcomes. Excessive methylation over-stabilises the duplex, which compromises the flexibility necessary for RNase-H to bind the RNA: DNA heteroduplex and results in incomplete target cleavage. In contrast, insufficient methylation results in residual immunogenicity and a decreased tissue half-life, leading to higher dosing frequency and therefore cumulative drug exposure with associated protein-binding-related toxicities. The middle ground, often observed with 60–70 % 2'-OMe content in the wings, provides the highest affinity and conformational breathing space necessary for catalytic degradation of the target sequence. Limited phosphorothioate at the termini was added to increase serum protein binding but without restoring the pro-inflammatory properties seen with the first generation of drugs. The final gapmer is thus a compromise that still maximises the therapeutic index and has resulted in several approved drugs with no detectable safety issues even with chronic dosing.
Industrial scale manufacture of 2'-O-methyl nucleotides requires a synthesis that is compatible with anhydrous alkylation conditions, which delivers a stereochemically pure product and which can be frozen under cGMP change-control before the first batch for toxicology studies is made. The additional step of methylation both elongates the synthetic tree, increases the solvent requirement and introduces an additional class of impurities (regio-isomeric sugars, residual alkoxides, trace fluoride) that need to be removed prior to release of the monomer as a qualified phosphoramidite. Success in this regard is therefore dependent on early consideration of the robustness of the protecting groups to moisture, and a mapping of the fate of impurities, so that the final multi-kilogram batches can be made with an impurity envelope identical to that of the gram-scale toxicology lot.
Introduction of the 2'-O-methyl group regioselectively, however, demands temporary protection of the 3'- and 5'-hydroxyls, typically with a bis-silyl or orthoester intermediate that opens under rigorously anhydrous conditions to generate only the 2'-alkoxide. Methylation is achieved using sodium hydride and methyl iodide or dimethyl sulfate. Both reagents are moisture-sensitive and the resultant metal salts can complex with the product and poison subsequent phosphitylation steps. Furthermore, any remaining water hydrolyses the activated sugar, creating 2',3'-mixed ethers that co-crystallise with the target isomer and are chromatographically silent. An additional complication is that if the base is itself protected with an acyl protecting group, the strong base required for deprotonation will induce acyl migration from the nucleobase to the sugar, creating N→O shift products which survive global deprotection and show up as mutagenic impurities in the API. Route scouting therefore involves forced-spiking studies to demonstrate that each intermediate can withstand the most challenging conditions of the following step without creating a new peak above the genotoxic qualification threshold during scale-up. Protecting groups are selected for orthogonal lability (fluoride-labile silyls for 2'-OH, base-labile amides for base amines, and acid-labile DMT for 5'-OH) such that the methylation step can be carried out before base-protection without cross-reactivity. The methylation solvent (THF or DMF) is then dried to <50 ppm water and must be filtered through activated alumina to remove trace peroxides that can oxidise the anomeric centre, a concern which magnifies when the reaction is performed in 1000 L reactors in which solvent turnover is slow and thermal gradients large.
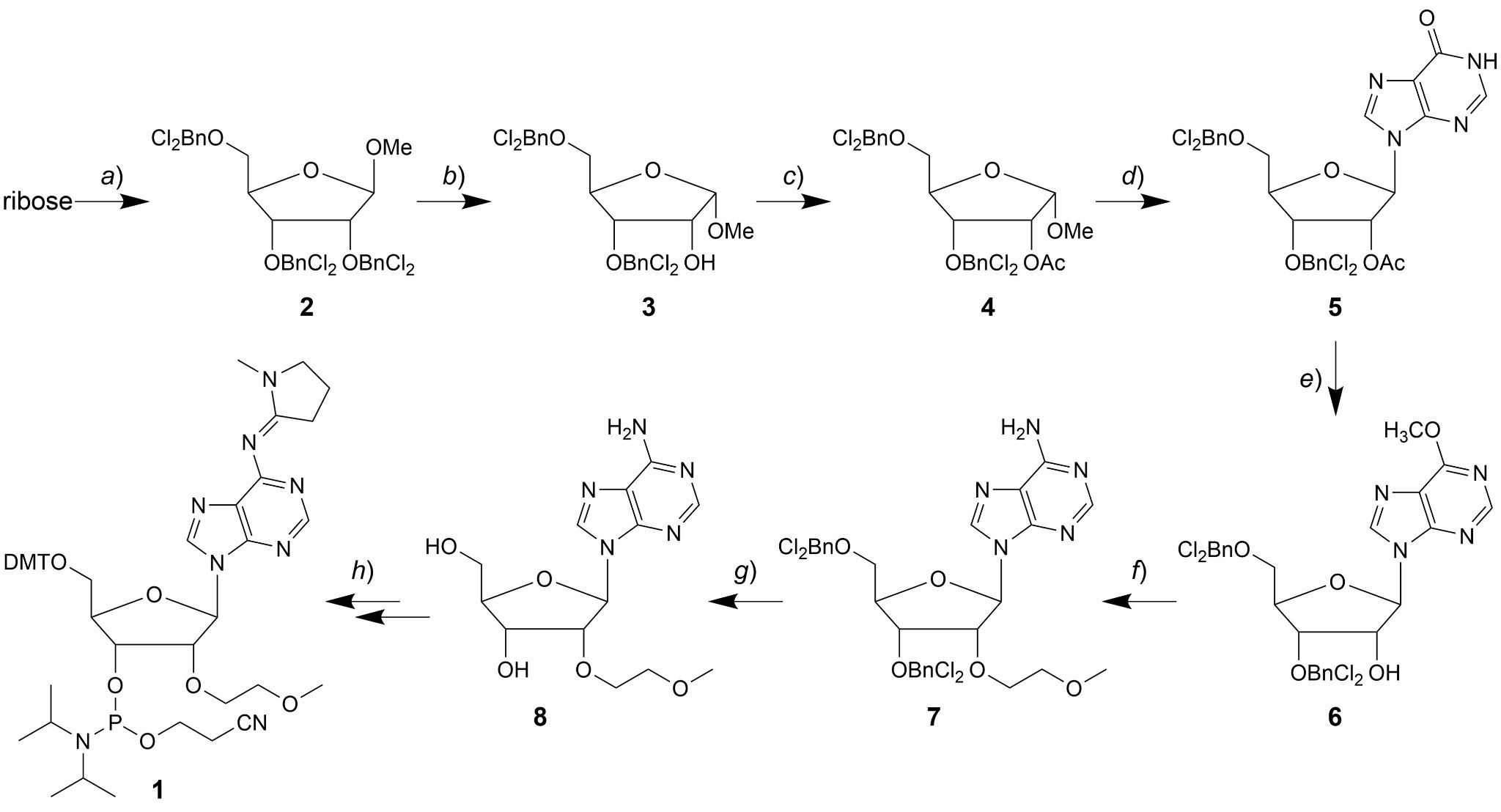 Fig. 2 Synthesis of the MOE modification.2,5
Fig. 2 Synthesis of the MOE modification.2,5
The qualified monomer should be 2'-epimerically pure (i.e., free of regio-isomeric sugars), as well as free of residual metal ions and moisture (exceeding the level that hydrolyses phosphoramidite during the stipulated shelf-life), all within the specification limits. The specification limits are confirmed by forced-degradation studies, in which the protected nucleoside is stored at elevated temperature (at 75 % relative humidity), to prove that the protecting-group integrity does not exceed acceptance criteria, for at least one year. The moisture content is determined by Karl-Fischer coulometry under inert gas: trace water levels can hydrolyze the amidite function as soon as the monomer is dissolved in anhydrous acetonitrile, hence a two-tier limit is set (closed-vial ppm and in-use ppm), to mimic the situation on the factory floor. The residual metals (Na, I, Al) introduced during the methylation or column chromatography, are profiled by ICP-MS: any of these ions in ppb levels can catalyze silyl migration or phosphate oxidation in subsequent coupling cycles. A stability-indicating HPLC method, using a dual-column system (ion-pair and hydrophilic interaction), should be able to resolve the authentic side-products (3'-O-methyl isomers or base-acyl adducts); new peaks appearing in excess of 0.1 % area under stress conditions should prompt a re-validation of the synthetic route. The monomer is packaged in aluminium-laminated pouches, back-flushed with argon, over-wrapped with desiccant packs; real-time moisture ingress is tracked by near-infrared spectroscopy, to ensure that the critical water activity remains below the validated ceiling, for the full labelled shelf-life.
Upscaling 2'-O-methyl nucleosides to multi-ton campaigns must be made in continuous-flow or loop-type reactors to avoid water formation. Efficient mixing is required to dissipate the exotherm arising from metal-hydride deprotonation. Batch-mode vessels >2 m3 are unsuited for this purpose, as local hot-spots cannot be removed from the reactor. Local heating may anomerise the sugar or cleave silyl ethers and thus, plug-flow tubular reactors with inline static mixers are well-suited for the methylation step. The subsequent work-up (quench-extract-crystallize) train is in a series of stainless-steel vessels qualified for explosive-proof operation as methyl iodide vapours can build-up under the nitrogen blanket to maintain anhydrous headspace. Solvent recovery streams are integrated: THF is distilled under nitrogen and passes through molecular sieve columns prior to direct reuse. Re-use of THF lowers cost and environmental impact. Change-control governance ensures that the synthetic route and crystallization solvent and drying protocol are locked-in under version control. If a change is required (for example, a different filter aid or dryer temperature), a formal comparability study is triggered including side-by-side phosphitylation to show that amidite yield and impurity profile remain within validated specifications. The GMP batch record also captures critical in-process checkpoints (methylation endpoint (NMR), moisture level after vacuum drying, and metal content prior to release) which ensures that each lot can be fully-traced back to raw-material receipts and forward-traced to final oligonucleotide drug substance.
Table 2 Process risk matrix for 2'-O-methyl scale-up
| Unit Operation | Critical Parameter | Failure Mode | Control Strategy |
| Metal-hydride deprotonation | Moisture | Side-product diol | Inline NIR, argon blanket |
| Methyl iodide charge | Temperature | Anomerisation | Jacketed plug-flow, ΔT <5 °C |
| Work-up quench | pH drift | Acyl migration | Buffered extract, real-time pH |
| Crystallisation | Solvent ratio | Mixed solvate | VT-XRD, seed loading |
| Vacuum drying | Vacuum level | Hydrolysis | 0.1 mbar, moisture trending |
We offer a comprehensive range of 2'-O-methyl (2'-OMe) nucleotide products and technical services to support the development and manufacturing of siRNA and antisense oligonucleotide therapeutics. By combining high-quality modified monomers with deep expertise in oligonucleotide chemistry, we help customers improve RNA stability, potency, and safety while ensuring reliable synthesis performance and scalable supply.
Our portfolio includes a full set of 2'-O-methyl-modified nucleotides covering all canonical RNA bases. These 2'-OMe nucleotides are widely used in siRNA and antisense drugs to enhance nuclease resistance, improve duplex stability, and reduce innate immune activation. All products are manufactured to high purity standards with tightly controlled impurity and moisture profiles, ensuring consistent performance during oligonucleotide synthesis. Our broad 2'-OMe offering enables flexible modification strategies, allowing developers to fine-tune sequence design to meet specific therapeutic and delivery requirements.
To support efficient chemical synthesis, we supply protected 2'-O-methyl nucleoside monomers that are fully compatible with phosphoramidite chemistry and automated solid-phase oligonucleotide synthesis. These monomers incorporate well-established, orthogonal protecting group systems that ensure controlled reactivity and clean deprotection. Our protected 2'-OMe monomers are designed to deliver high coupling efficiency, reduced side reactions, and consistent sequence fidelity, even in highly modified siRNA and antisense constructs. This synthesis-ready design helps manufacturers maintain robust and reproducible production processes.
For programs requiring specialized modification patterns or non-standard designs, we provide custom synthesis and process optimization support for 2'-O-methyl nucleotides. Our technical team assists with modification placement strategies, protecting group selection, synthetic route optimization, and impurity control. By considering both molecular design and manufacturability, we help ensure that custom 2'-OMe nucleotides are scalable, reproducible, and suitable for long-term development, reducing technical risk as programs advance toward clinical and commercial stages.
We offer GMP-ready supply options for 2'-O-methyl nucleotides to support clinical and commercial manufacturing of siRNA and antisense drugs. Materials are provided with clear specifications, certificates of analysis, traceability, and change control documentation, supporting regulatory expectations and audit readiness. This regulatory-focused supply approach enables smooth transitions from research to GMP production while ensuring consistent quality and long-term supply continuity.
If you are developing siRNA or antisense oligonucleotide therapeutics that require high-quality 2'-O-methyl nucleotides, our team is ready to support your program. Contact us today to request technical information, samples, or quotations, or to discuss custom 2'-OMe nucleotide solutions and GMP-ready supply options tailored to your development needs.
References
It improves stability and reduces immune activation.
Yes, especially for off-target and safety control.
They often improve binding without compromising activity.
Yes, particularly in steric-blocking ASO designs.
Yes, they require specific protecting strategies.

