DNA, RNA, and analogue strands are created through solid-phase phosphoramidite synthesis which links nucleoside and nucleotide monomers in sequence as sugar-bound bases or base-phosphate sugar units. As each coupling step must be near quantitative to prevent truncation, the monomers must be of high purity, have defined stereochemistry and controlled moisture content; any base-deprotected impurity or residual acid is incorporated and propagates to full-length truncated sequences, impacting drug efficacy, off-target hybridization and regulatory impurity specifications.
Oligonucleotide (oligo) synthesis is done chemically via a phosphoramidite-based step-wise elongation process, using nucleoside building blocks that are added in a specific order to therapeutic DNA or RNA strands. A cycle of deprotection, coupling, oxidation and capping reactions are carried out for each addition on a solid support. Purity, anomeric fidelity and chemical modification of the incoming monomers determine binding affinity, nuclease resistance and clinical potency of the resulting drug. As a result, the raw-material specification step is the first critical control point in oligo manufacturing, predating sequence design or delivery formulation.
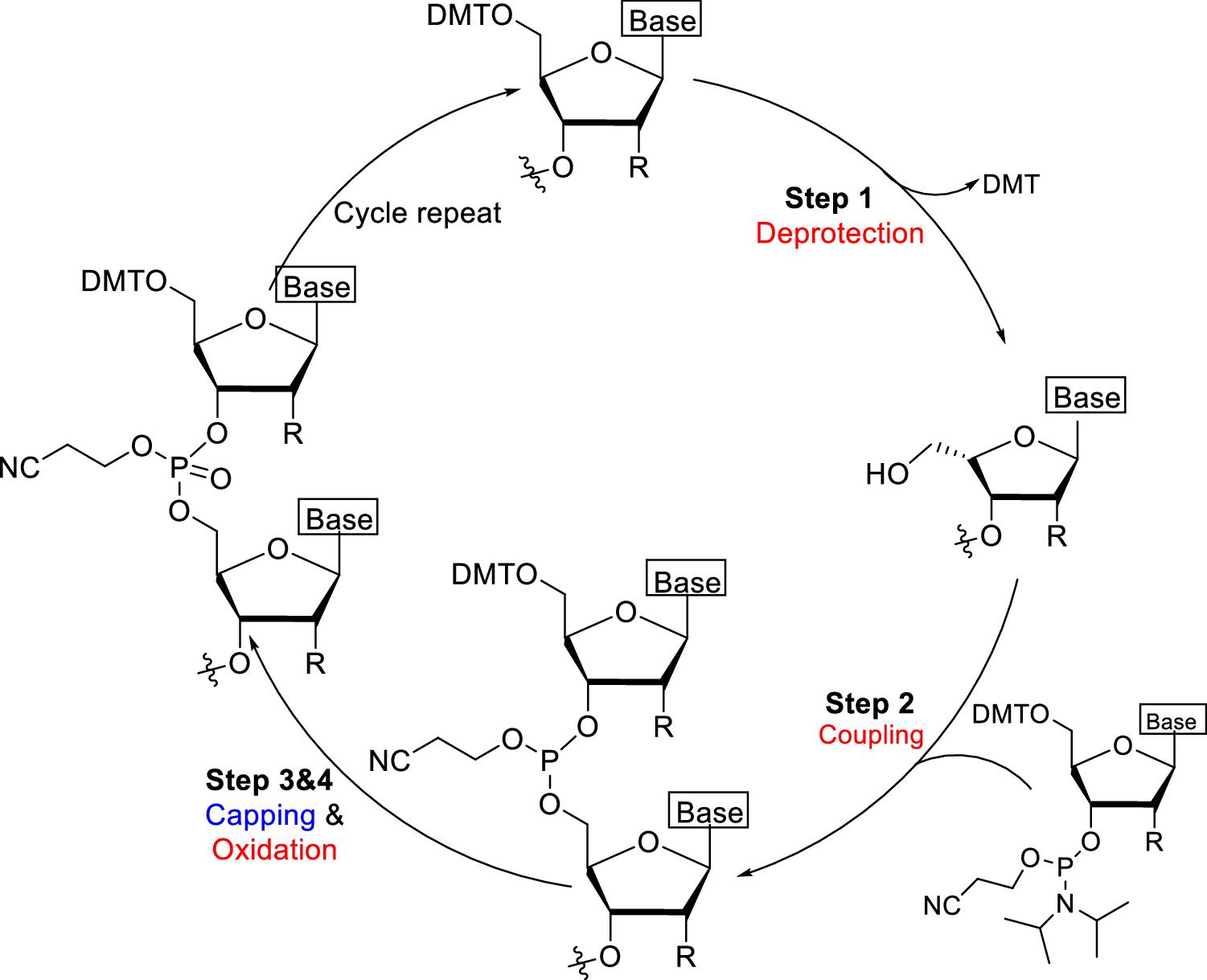 Fig. 1 A generalized synthesis cycle of an oligonucleotides through phosphoramidite approach.1,5
Fig. 1 A generalized synthesis cycle of an oligonucleotides through phosphoramidite approach.1,5
Trace impurities in phosphoramidites cause single-base deletions or anomeric inversions, the former of which abolishes activity (gene silencing) and the latter of which can create potent off-target immunostimulation. For this reason, high-quality monomers have very strict QC: chiral purity >99.5 %, water content<50 ppm, and absence of trace metals that can catalyze depurination. Furthermore, with modified monomers (2'-F, phosphorothioate), these modifications need to be in the correct C-2' or C-3' stereochemistry for RNA-like binding affinity and nuclease resistance. In the absence of these specifications, even perfectly designed sequences fail in vivo.
DNA monomers are the usual 2'-deoxyribosyl phosphoramidites 5'-protected as DMTs and 3'-protected as β-cyanoethyl. RNA monomers have 2'-O-TBDMS or 2'-O-MOE protecting groups, in addition to DMT/β-cyanoethyl, to prevent ribonuclease cleavage. Therapeutic oligos are often chemically modified to enhance affinity to RNA. All such chemical modifications are introduced as part of the monomer (building-block) strategy. The building-block strategy has the advantage that a number of chemistries can be introduced in one step, so that siRNA, mRNA, or antisense strands with specific pharmacokinetics can be synthesized without post-synthetic modification.
Examples of such libraries include the 2'-F and 2'-O-MOE monomers used in siRNA to increase duplex Tm while decreasing off-target effects, N1-methyl-pseudouridine monomers used in mRNA to dampen innate immune sensing while preserving translation, phosphorothioate monomers used in antisense oligos to impart serum stability and recruit RNase H, and LNA or 2'-F monomers used in aptamers to bestow picomolar affinity. By this token, a single monomer library may support a wide range of therapeutic modalities, only distinguished by monomer choice as opposed to post-synthetic chemistry.
Table 1 Monomer requirements for major oligonucleotide drug classes
| Drug Class | Sugar Type | Key Monomer Modification | Functional Goal |
| siRNA | Ribose | 2'-O-methyl A/U | Reduce TLR7/8 activation |
| mRNA | Ribose | N1-Me-Ψ | Increase translation |
| Antisense | Deoxyribose | Phosphorothioate | Serum stability |
| Aptamer | Ribose | 2'-F, LNA | High affinity |
Nucleosides and nucleotides are the fundamental structural entities from which all oligo drugs are built. A nucleoside is defined as a nitrogenous base (purine or pyrimidine) bound to a ribose or, and a nucleotide is a nucleoside with an additional phosphate group bound to the sugar. As each cycle of the solid phase synthesis involves incorporation of a phosphate linkage, the monomer loaded into the instrument is in practically all cases a nucleoside phosphoramidite. This has the consequence that the synthetic process begins with a nucleoside and terminates with a nucleotide polymer.
A nucleoside is a 5-carbon sugar attached to a planar heterocycle: the purines adenine (A) and guanine (G), and the pyrimidines cytosine (C), thymine (T) and uracil (U). The C-1' of the sugar is attached to the N-9 (purines) or N-1 (pyrimidines) position of the base by a β-N-glycosidic bond, and fixes the stereochemistry which will determine helical twist and base-pair geometry in the final oligo. The presence of a hydroxyl at the 2'-carbon of ribose confers the A-form geometry required for RNA duplexes; the absence of the -OH on deoxyribose results in the wider, more stable B-form helix of DNA.
The addition of one (or three) phosphate group(s) converts the nucleoside into a nucleotide: 5'- NMP, NDP or NTP. Both ATP and GTP function as universal biological energy currencies though nucleoside triphosphates play a role in enzymatic RNA polymerization for mRNA production during oligo synthesis whereas nucleoside phosphoramidites serve as monomers in solid-phase chemical synthesis of antisense sequences together with siRNA and aptamer molecules. The cell phosphorylates nucleosides stepwise using kinases; the chemist adds the phosphate all at once as a protected amidite. While the terminal phosphodiester bonds in DNA or RNA molecules share similar chemical properties whether produced biologically or synthetically, the method of synthesis determines the specific type of monomer building block to purchase for synthesis.
Adding the phosphate ester to the 5'-carbon creates the sole structural difference which significantly changes charge and polarity as well as metabolic fate. Nucleosides diffuse through membranes due to their neutral and lipophilic properties while nucleotides require transport mechanisms because of their negative charge. During oligo synthesis the phosphate undergoes temporary masking with a phosphoramidite to facilitate solid-phase coupling which is followed by oxidation that transforms the phosphodiester backbone into the structure that provides strand rigidity and charge density for cellular uptake and base-pair recognition.
The synthesis of oligos requires precise chemical control to polymerize nucleoside or nucleotide monomers into longer chains. The solid-phase phosphoramidite method starts with nucleoside substrates protected by masking groups and reactive phosphoramidite units which enable the formation of phosphate linkages during the coupling-oxidation cycle that integrates each nucleotide into the chain. The process of synthesizing nucleotide polymers from nucleoside precursors requires monomer selection to define the polymer backbone, determine coupling effectiveness and impurity outcomes while core instrumentation remains unaffected.
Protected nucleosides (5'-DMT, 3'-β-cyanoethyl phosphoramidite) are readily activated monomers that can be coupled in<45 s="" per="" cycle="" at="">99 % step yield. They are small, so a high loading can be achieved on CPG or polystyrene support, and their orthogonal protecting groups (DMT for 5'-OH, β-cyanoethyl for phosphate) avoid side reactions. The use of nucleosides as starting material rather than pre-formed dinucleotides allows for single-base precision, iterative changes to the sequence, and for the incorporation of therapeutic modifications (2'-F, PS, LNA) during the elongation phase, which makes regulatory CMC easier and also reduces the synthetic complexity.
Each monomer is supplied as a β-cyanoethyl phosphoramidite: 3'-phosphite couples to the 5'-OH of the growing chain, then iodine oxidation P(III)→P(V) yields a phosphodiester or phosphorothioate backbone. The β-cyanoethyl protecting group is removed with aqueous ammonia after synthesis to give a natural backbone. This chemistry also provides single-nucleotide precision, >99 % step yield, and the capacity to incorporate therapeutic modifications (phosphorothioate, 2'-F) during chain elongation, avoiding a post-synthetic modification step.
DNA monomers do not have a 2'-OH, and so require only base and 5'-OH protection. RNA monomers have a 2'-OH, which must be masked (commonly with TBDMS or TOM groups) to prevent side reactions and to maintain A-form helix geometry. This additional protection adds to the cycle time and necessitates more robust deprotection cocktails, but is crucial for siRNA or aptamer drugs for which the RNA-like structure is a key determinant of potency. The steric bulk of the 2'-O-silyl group reduces coupling rates by ~10 % vs DNA; thus, RNA syntheses often use more reactive amidites (such as 2'-O-TOM) and longer coupling times. After synthesis, fluoride-mediated desilylation is also necessary, an additional step not required for DNA; hence the same synthesiser can be used for both backbones, but RNA protocols require sugar-specific monomers, and post-synthetic care to prevent 2'-→3' phosphate migration or strand scission.
Therapeutic oligos are constructed from nucleoside monomers spanning from canonical DNA/RNA backbones to fully modified and highly engineered analogs. Native monomers determine the base-pairing alphabet, while sugar or base modifications can be tuned to modulate nuclease resistance, duplex stability and immunogenicity. As each amidite is sequentially appended in order, the choice of monomer (native 2'-deoxy, 2'-O-methyl, 2'-MOE, 2'-F, phosphorothioate, etc.) is encoded at the feedstock stage, allowing a single solid-phase synthesizer to oscillate between antisense, siRNA, aptamer or mRNA templates with no change to core chemistry.
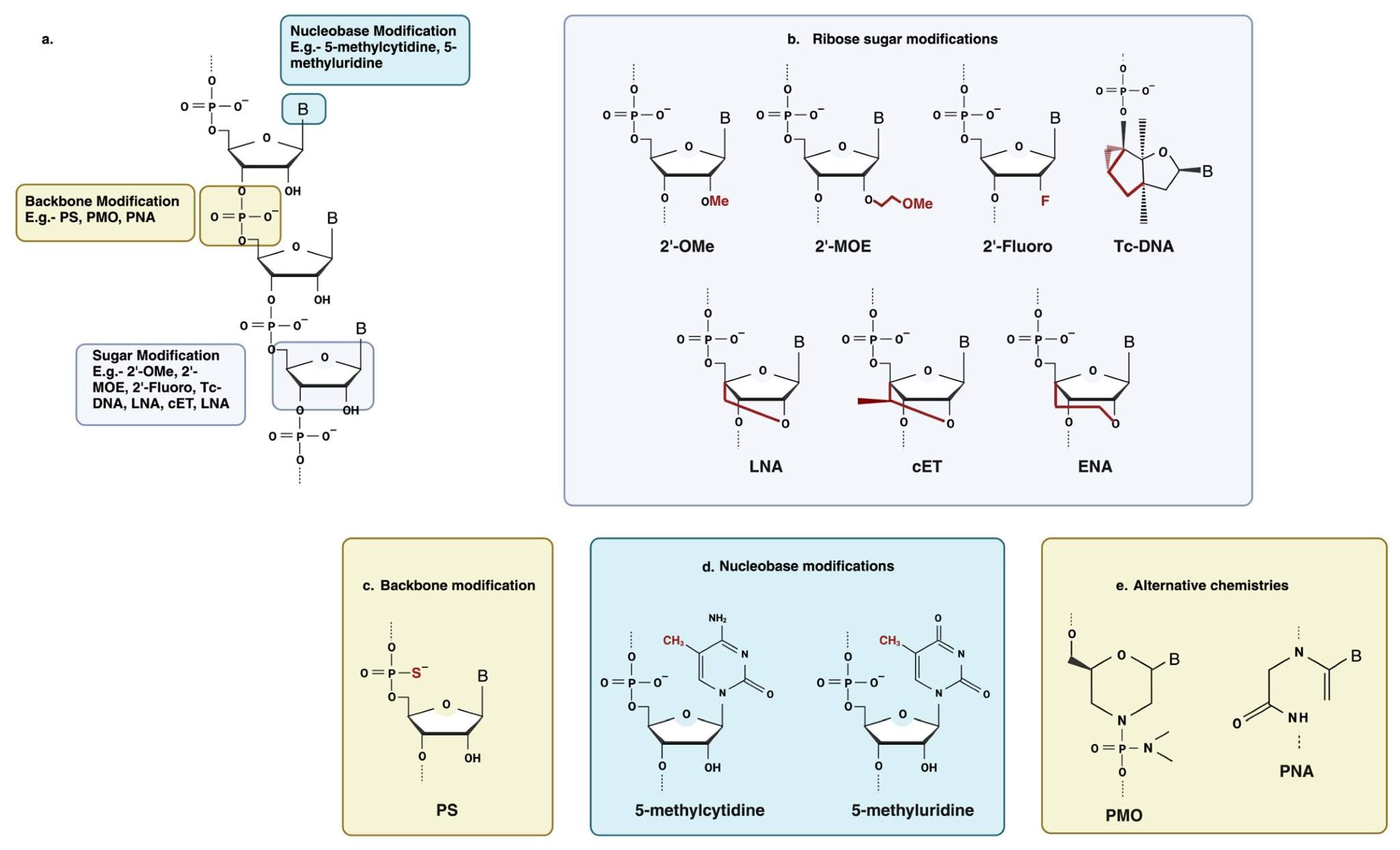 Fig. 2 Some common chemical modifications used in antisense oligonucleotide chemistry.2,5
Fig. 2 Some common chemical modifications used in antisense oligonucleotide chemistry.2,5
Synthesis of oligos is initiated from the nucleoside phosphoramidites of A, T/U, C and G. The primary structural difference between DNA and RNA monomers is the presence of only hydrogen substitution at the 2'-carbon (deoxyribose) in the case of DNA monomers, while the 2'-OH (ribose) is retained in the case of RNA monomers. The latter require an additional protecting group(s) (TBDMS or ACE) for the 2'OH to prevent 2'-3' migration during the solid-phase coupling. These canonical units are still employed when maximum compatibility with polymerases is desired, but they are rapidly degraded by serum nucleases, and thus chemical embellishment is necessary for in vivo applications.
2'-O-Me enforces the C3'-endo RNA conformation, raises duplex Tm by ~1 °C per residue, and sterically blocks 2'-OH mediated strand cleavage. 2'-O-Me is the naturally-occurring nucleotide modification in tRNA, and it is commonly incorporated into guide strands of siRNA to minimize off-target effects while still recruiting RNase H upon pairing with DNA. 2'-F locks the sugar into C3'-endo conformation, enhancing affinity and nuclease resistance without appreciably raising toxicity. It is broadly used in both guide and passenger strands of siRNA, as well as aptamers and antisense oligos for high-affinity binding while retaining A-form helix geometry. 2'-O-methoxyethyl (MOE) is a flexible, hydrophilic ether extension that elongates the 2'-oxygen by two atoms. This simple addition multiplies steric bulk by orders of magnitude and nearly triples serum half-life. The ether oxygen can form hydrogen bonds with water to maintain solubility while the ethyl group sterically protects the ribose from nuclease access. Antisense drugs that use MOE monomers as the monomer of choice are known as gapmers because they recruit RNase H while leaving terminal regions immune-silent and stable.
Base analogues (5-methyl-C, 5-bromo-U, 7-deaza-A) that do not change the Watson-Crick faces but have high stacking energies and low immunogenicity. In particular, 5-methyl cytidine (a common modification that mimics endogenous DNA methylation) makes the strand much less visible to TLR9, while also significantly boosting duplex Tm. 7-deaza modifications remove the N-7 lone pair that can be protonated at low pH, preventing depurination during long-term storage at low pH or exposure to lysosomal conditions. Since changes to bases are orthogonal to those to sugars, they can be combined with 2'-MOE or phosphorothioate linkages to make "fully modified" strands, which resist every major degradation pathway.
Collectively, sugar and base modifications dramatically prolong serum half-life from minutes to hours and thereby enable more widely spaced dosing. Higher Tm also helps with strand invasion into structured mRNA, which can improve antisense potency. The "immune-quiet" sugars 2'-O-Me and 2'-F also avoid TLR7/8 activation and reduce flu-like side-effects. Finally, lipophilic modifications, such as 2'-MOE, can improve binding to plasma proteins, thereby slowing renal filtration, and increasing tissue exposure without increasing peak plasma concentration, a desirable pharmacokinetic profile that couples potency with safety.
Table 2 Functional impact of common nucleoside modifications
| Modification | Location | Key benefit | Typical use |
| 2'-O-Me | Sugar | Nuclease block, ↑Tm | siRNA passenger, aptamer |
| 2'-F | Sugar | ↑Affinity, immune quiet | siRNA guide, anti-miR |
| 2'-MOE | Sugar | Long t1/2, RNase H gap | Antisense gapmer wings |
| 5-Me-C | Base | TLR9 quiet, ↑stack | Antisense, CpG dampening |
| PS linkage | Backbone | Serum stability | All therapeutic classes |
Therapeutic oligos are synthesized from protected monomers (nucleosides or nucleotides). All the reactive hydroxyls, amines and phosphates are protected by temporary chemical "curtains" that prevent side reactions and ensure fidelity to a single base during the iterative coupling steps and are only removed after the complete sequence has been assembled. Since each coupling cycle must go to >99 % yield to avoid prohibitive truncation, the choice, lability and orthogonality of protecting groups directly control final drug purity, potency, and regulatory impurity profile.
Otherwise, the 5'-OH would react randomly, the exocyclic amines of A/G would get acylated, and the phosphate would branch or hydrolyze. So the protecting groups serve as temporary "mute" buttons: 5'-DMT for the 5'-OH, benzoyl or acetyl for the base amines, and β-cyanoethyl for the phosphate. The protecting groups are orthogonal - can be removed by different conditions - so that only the desired hydroxyl is left free for each coupling cycle. This permits single-base accuracy and avoids truncated or branched sequences.
Base protection: Benzoyl (on adenine and cytidine) and isobutyryl (on guanine) prevent N-acylation during activation of the amidite. Pac (phenoxyacetyl) groups are becoming popular for their ultra-mild deprotection conditions, which help prevent depurination in RNA or base-modified strands. Sugar protection: DMT at the 5'-OH is lipophilic, orange-colored and photo-labile. It allows monitoring of coupling yield in real time. When making RNA, the 2'-OH is protected by TBDMS or TOM groups, which are bulkier, so more reactive versions of the standard amidites are used, with longer coupling times to maintain similar yields. Phosphate protection: β-Cyanoethyl (CE) esters protect the phosphate. They are removed by a mild base (aqueous ammonia) during final cleavage, leaving behind the natural phosphodiester. Methyl or allyl esters are used when ultra-mild conditions are needed, to protect base-sensitive modifications.
Bulky 2'-O-TBDMS decelerates coupling ~10 %; therefore, 2'-O-TOM or ACE groups with electron-withdrawing substituents are used to compensate for reactivity loss. Photo-cleavable or fluoride-labile protecting groups allow orthogonal removal under neutral conditions, minimizing depurination and increasing full-length product from ~75 % to >90 %. Because deprotection is the final step, any residual protecting group becomes an extractable impurity; therefore, lability and wash protocols are validated to satisfy ICH Q3A limits, directly tying protecting-group chemistry to regulatory success.
Nucleoside monomers: the "bare-bones" scaffolds (base + sugar) that need to be chemically dressed before they can be loaded into a solid-phase oligo synthesiser. The dressing step adds a 3'-O-phosphoramidite group, which converts the nucleoside into an amidite building block that is stable during storage, but is instantly activated by weak acid to form a P(III) intermediate for step-wise coupling. As this transformation is performed on the nucleoside before polymerisation, the same parent scaffold (adenosine, cytidine etc.) can be used to make DNA, RNA, 2'-F, 2'-O-Me or phosphorothioate linkages simply by selecting which protecting groups and phosphate masks are added at the monomer stage.
The conversion is a two-step, one-pot anhydrous protocol. The 5'-OH of the nucleoside is first masked with dimethoxytrityl (DMT) chloride. Second, a phosphitylating reagent (usually chloro-N,N-diisopropylamino-phosphine) is reacted with the 3'-OH in the presence of a mild base (DIPEA). The resulting phosphoramidite has a β-cyanoethyl group on the phosphate and diisopropylamino on the phosphorus. It is an air-stable white solid, which can be stored for months, and which couples in >99 % yield when activated with tetrazole. Because the reaction is done on protected nucleosides, base-labile acyl groups (benzoyl, isobutyryl) are not disturbed. Thus, only the 3'-OH is phosphorylated, and no regio-isomeric mixture is produced.
Solid-phase synthesis then proceeds through the cycle of DMT deprotection → tetrazole activation → coupling → oxidation → capping. The activated amidite is a phosphite triester that reacts with the 5'-OH of the immobilised strand to form the phosphite linkage, which is immediately oxidised to a phosphotriester (or phosphorothioate if being sulfurised). Because the amidite is a P(III) species, the reaction is fast (30–60 s) and driven to near-completion under mild conditions, allowing single-base resolution for antisense, siRNA or aptamer sequences without enzymatic bias. Because the same cycle is repeated for every addition, the number of cycles equals the final chain length, giving chemical synthesis an advantage over polymerase-based methods for short, precisely modified strands.
The industry standard status is due to the unrivalled coupling efficiency, modularity and scalability. Activated phosphoramidites react in<30 s at room temperature, are tolerant of a wide range of bases and sugars (including 2'-O-methyl, LNA or phosphorothioate) and can be dispensed by automated synthesizers that handle everything from milligram research quantities to multi-kilogram GMP campaigns. Alternative chemistries (H-phosphonate, phosphotriester) require harsher conditions or multiple oxidation steps, which leads to lower yields and more impurities. Finally, the phosphoramidite route integrates seamlessly with solid-phase resins, enabling iterative addition without intermediate purification, a workflow that no other chemistry has yet matched.
The quality of the final product is encoded into the monomers of an oligo. Purity, moisture, residual solvents, metal ions and stereochemical integrity must therefore be specified and controlled before the first coupling cycle. Because each amidite addition is essentially irreversible, any truncation or side product generated from an out-of-spec monomer is propagated and amplified, directly controlling the final full-length yield, impurity profile and regulatory acceptability. The monomer is thus treated as a drug substance in miniature, to which ICH Q7 controls are applied, despite its apparently simple chemical nature.
Milligram scale synthesis, or screening, can use research grade amidites, which are typically ≥98 % as determined by HPLC. But GMP lots must be ≥99.5 % (HPLC) in chemical purity, and ≥99 % (chiral HPLC) in single-isomer purity (phosphorus diastereomer). The latter condition ensures that only one diastereomer at phosphorus proceeds. Trace depurination products can seed unwanted immunotoxic truncations, so lots are also screened for the presence of low-molecular-weight UV-active impurities by LC-MS. Finally, to link monomer quality to final drug safety, a spiking-recovery challenge is performed: the monomer is deliberately spiked with a known impurity, synthesized, and analyzed to demonstrate that the spiked impurity is either rejected or removed by downstream purification.
Phosphoramidite chemistry is extremely moisture-sensitive: >200 ppm hydrolyses the P(III) centre, reducing coupling efficiency and forming P-chiral diastereomers that show up as " +1 " peaks in MS. Karl-Fischer titration is therefore performed on every batch, with a release spec ≤100 ppm. Residual solvents (dichloromethane, toluene, pyridine) are quantified by GC-MS against ICH Q3C limits; even ppm levels of pyridine can quench the activation acid, so Class 2 solvents are maintained at ≤50 ppm. Transition metals (Fe, Cu, Zn) catalyse oxidative depurination; ICP-MS is used to show ≤5 ppm total metals, so that the monomer will not seed downstream strand breaks or colour-forming impurities during long-term storage.
For regulatory submissions, the control strategy is designed to make connections between monomer critical quality attributes (CQAs) and ultimate oligo performance. As part of this, purity, moisture and metal data are statistically trended across a number of consecutive batches to demonstrate process capability (Cpk ≥1.33). Since the amidites are typically procured from multiple vendors, qualification requirements ensure each has equivalent impurity profiles and spiking-recovery performance. A shelf-life specification (typically 24–36 months at –20 °C under argon) is determined by accelerated stability studies, which prove that moisture uptake, isomer ratio and UV purity remain within release limits, so batch-to-batch consistency is tied to long-term drug product stability.
1H-NMR offers structural identity and detection of residual solvents; 31P-NMR the correct oxidation state (P(III) vs P(V)) and, in combination with HPLC methods, detection of diastereomeric drift. LC-MS is used for structural identity and impurity "fingerprints". Chiral HPLC can be used to quantify the Rp/Sp ratio at phosphorus. FT-IR can serve as a fingerprint for functional groups (P=O, C=O). DSC can be used to confirm melting behaviour and thereby confirm identity and rule out polymorphic impurities. Since the analytical methods available are orthogonal in nature, a multi-attribute specification is applied: identity (NMR), purity (LC-MS), water (Karl-Fischer), metals (ICP-MS) and residual solvents (GC-MS), resulting in a complete analytical package where the monomer is treated as a fully characterized drug substance.
Within the modern oligo therapeutic space, nucleoside monomers are programmable chemical pixels: altering sugar pucker, heterocycle, or internucleotide linkage leads to unique gene knockdown, splicing repair, or translational enhancement phenotypes. This synthetic syntax (protection/deprotection cycles, phosphoramidite coupling, orthogonal base editing) is shared by siRNA duplexes, gapmer ASOs, mRNA vaccines, aptamer folds, and emerging circular/branched RNA architectures. It allows a single medicinal-chemistry platform to target liver, muscle, CNS, and ocular disease indications without re-inventing route chemistry.
Helper lipids like DSPC and cholesterol, used in siRNA-lipid nanoparticles to provide bilayer stability, and the cone-shaped DOPE, required for endosomal escape, ensure that the ionizable cationic lipid releases the siRNA payload into the cytosol to allow RISC loading. Glycolipids can be added into this same particle. The carbohydrate head-groups on the glycolipid are then processed by dendritic cells which detect the glycolipid through TLR4. This leads to the release of Type-I interferon, which promotes silencing of the siRNA cargo, without increasing off-target activity of the passenger-strand. As the same ceramide-backbone is maintained throughout this process, synthetic complexity is locked to ≤ 3 linear steps from commodity plant sterols. This means that adjuvant optimization must be done within the existing CMC workflow, rather than a parallel development path.
Table 2 Helper Lipid Functions in siRNA-LNP Systems
| Lipid Component | Primary Role | Glycolipid Addition | Functional Gain |
| Ionizable lipid | Endosomal escape | Co-encapsulation | Adjuvanticity |
| DSPC | Bilayer rigidity | Raft stabilization | Particle integrity |
| DOPE | Fusogenicity | Cone geometry | siRNA release |
| Cholesterol | Membrane order | Curvature tuning | Biodistribution |
ASOs exploit Watson-Crick hybridization to target RNase H or sterically block splicing. Naked phosphorothioate backbones, however, are rapidly renally cleared and exhibit off-target hybridization. Glycolipid conjugation, using a ceramide linker to the 3'-end, anchors the ASO into lipoprotein particles, prolonging circulation half-life and biasing uptake to liver sinusoidal endothelial cells that express SR-BI and CD1d. Presentation of the ceramide-ASO conjugate on CD1d activates iNKT cells, releasing IFN-γ which up-regulates RNase H1 expression, amplifying target mRNA degradation without increasing oligo dose. The conjugate is metabolized into natural sphingolipids once the ceramide tail is cleaved by acid ceramidase, avoiding accumulation of synthetic linkers that can activate innate sensors such as TLR7.
Glycolipid-conjugated cap analogues can be co-transcriptionally loaded into mRNA during in-vitro transcription. The lipid tail anchors the transcript to liposomal membranes forming a self-adjuvanting RNA particle. The ceramide moiety is recognized by CD1d on dendritic cells, functioning as a danger signal and eliminating the need for separate adjuvants like lipid A or poly(I:C). Ceramide conjugation at the 5'-end of self-amplifying RNA replicons stabilizes secondary structure near the ribosomal entry site, enhancing translational efficiency, and also functions as an iNKT agonist. Emerging circular RNA platforms can also use ceramide-PEG conjugates for membrane anchoring and to hide nuclease binding sites, providing longer functional half-lives without interfering with the circular topology needed for protein expression.
Aptamers require 3D folding to recognize their target, but naked DNA or RNA aptamers are quickly degraded by serum nucleases and eliminated by renal filtration. Conjugation of glycolipid anchors (using a cholesterol-ceramide hetero-bifunctional linker) inserts aptamers into the membrane of lipoproteins or extracellular-vesicles, prolongs circulation half-life, and tunes the uptake mechanism to favor expression of the target protein and CD1d. For instance, anti-EGFR CL4 conjugated to ceramide-cholesterol tail is localized to lipid rafts where EGFR is located, which enhances binding avidity and iNKT-mediated immune activation without off-target uptake. Custom nucleotide monomers with 2'-fluoro or 2'-O-methyl substitutions can be co-polymerized with ceramide-phosphoramidite reagents in one-pot solid-phase synthesis of self-adjuvanting aptamers that act as both targeting ligand and immune activator (feature difficult to reproduce with protein-based aptamer conjugates).
We provide a comprehensive range of nucleoside and nucleotide monomers to support oligonucleotide synthesis across discovery, development, and commercial manufacturing. Our offerings are designed for RNA and DNA therapeutics, combining high-quality raw materials with deep technical expertise to help customers achieve reliable synthesis performance, efficient scale-up, and long-term supply continuity.
Our portfolio includes a full set of A, T/U, C, and G nucleoside derivatives tailored for both RNA- and DNA-based oligonucleotide platforms. We supply ribose and deoxyribose nucleosides to support applications ranging from siRNA and antisense oligonucleotides to mRNA therapeutics and aptamers. These nucleoside derivatives are produced under stringent quality standards to ensure high purity, low moisture content, and consistent batch performance. Native, selectively protected, and application-ready nucleosides are available to support efficient downstream conversion into activated intermediates, helping to maintain high coupling efficiency and sequence fidelity during oligonucleotide synthesis.
To address the growing complexity of modern oligonucleotide drugs, we offer a broad selection of modified and protected nucleoside and nucleotide monomers optimized for chemical synthesis. Our portfolio includes 2'-modified nucleosides such as 2'-O-methyl, 2'-fluoro, and 2'-MOE, as well as base- and sugar-protected monomers compatible with solid-phase synthesis. These materials are designed to deliver robust coupling performance, controlled reactivity, and clean deprotection, enabling the synthesis of high-quality oligonucleotides with improved stability, potency, and safety profiles. Our protected monomers support both early-stage research and advanced therapeutic manufacturing.
We supply phosphoramidite precursors and fully activated phosphoramidite building blocks that integrate seamlessly into automated oligonucleotide synthesis processes. These materials are optimized for consistent reactivity and reproducibility, supporting high-throughput and large-scale production. For specialized programs, we provide custom monomer development services, including novel base or sugar modifications, alternative protecting group strategies, and synthetic route optimization. Our team collaborates closely with customers to transform innovative molecular designs into scalable, manufacturable raw materials suitable for long-term development and commercialization.
Beyond material supply, we offer technical support throughout the oligonucleotide development lifecycle. Our experts assist with monomer selection, protecting group compatibility, coupling efficiency optimization, and impurity mitigation to help streamline synthesis workflows. As programs advance toward scale-up and GMP manufacturing, we provide guidance on process robustness, change management, and raw material qualification, helping customers reduce technical risk and avoid costly late-stage modifications.
We support oligonucleotide programs at every stage with flexible and scalable supply options. From small-quantity research materials to larger-volume GMP-ready supply, our nucleoside and nucleotide monomers are accompanied by clear specifications, traceability, and appropriate documentation. This flexibility enables a smooth transition from discovery through clinical development and commercialization, ensuring reliable access to high-quality raw materials as program requirements evolve.
If you are looking for high-quality nucleoside and nucleotide monomers backed by deep technical expertise, our team is ready to support your oligonucleotide program. Contact us today to request technical information, samples, or quotations, or to discuss custom monomer development and scale-up solutions tailored to your needs.
References
Nucleoside monomers are building blocks composed of a nucleobase and sugar, used as starting materials in chemical oligonucleotide synthesis.
Nucleotides contain one or more phosphate groups, while nucleosides do not, leading to different roles in synthesis and biology.
Nucleosides offer better chemical stability and compatibility with protecting group and phosphoramidite chemistry.
Nucleotides are mainly used in enzymatic processes like in vitro transcription, not solid-phase synthesis.
siRNA, antisense oligonucleotides, and mRNA therapeutics all depend on high-quality nucleoside monomers.

