High-purity adenosine derivatives serve as the purine backbone of therapeutic oligonucleotides: Their large π-surface reinforces base-stacking and the ribose or deoxyribose platform can be bridged, fluorinated or N-alkylated at the monomer level to encode nuclease resistance, duplex stability and innate-sensor evasion without impacting the amidite coupling cycle thereby enabling the same automated synthesizer to produce siRNA, mRNA or antisense drugs whose pharmacokinetics are pre-programmed at the adenosine level, a level of chemical determinism that streamlines lead-time across multiple RNA platforms while meeting a single, regulator-accepted manufacturing backbone.
Adenosine is unique in that it is the only nucleoside that codes information, fuels energy metabolism and has four orthogonal handles (N6, C8, 2'-OH, 5'-OH) for medicinal chemistry; as a result, high-purity derivatives can act as both messenger and modulator, and are a key building block for RNA and DNA drug modalities.
 Fig. 1 Chemical structures of adenosine derivatives.1,5
Fig. 1 Chemical structures of adenosine derivatives.1,5
Adenosine is the backbone of all clinically developed oligonucleotide platforms. In gapmer antisense, deoxyadenosine backbones anchor RNase H1 cleavage of huntingtin or SMN2 transcripts, with 2'-MOE-adenosine wings that increase binding affinity and confer exonuclease resistance. In siRNA, 2'-fluoro-adenosine rigidifies the guide strand seed region to optimize argonaute loading, and N6-methyl-adenosine at position 14 promotes RISC maturation while avoiding TLR7 activation. In mRNA vaccines, complete replacement by N6-methyl-pseudoadenosine and 5-methoxy-uridine abolish PKR and RIG-I signalling, to allow self-amplifying replicons to achieve monoclonal antibody expression at microgram-per-milliliter titers in serum. Aptamer libraries use 8-bromo-adenosine to freeze the glycosidic bond in the syn conformation, to stabilize G-quadruplex folds that bind heparin-binding growth factors with nanomolar affinity. Hence, adenosine edits - 2'-F, N6-Me, 8-Br - function as addressable chemical pixels to toggle catalytic, translational or quadruplex phenotypes on demand.
The clinical validation of siRNA for hereditary transthyretin amyloidosis, and of mRNA for prophylactic vaccines, has transformed adenosine derivatives from catalogue reagents to critical raw materials. Regulatory requirements for GMP-grade monomers with defined residual solvents, trace metals and microbial burden are driving suppliers towards continuous-flow amidite synthesis, on-line analytics and single-use bioreactors. Emerging modalities – self-amplifying RNA, circular RNA, and bispecific aptamers – will further fragment demand by asking for 5'-triphosphate analogues, 2'-amino-adenosines and lipid-conjugated bases that were until recently merely academic curiosities. The four-letter nucleoside alphabet is being rapidly transformed into a commodity chemical portfolio whose capacity planning, supply-chain resilience and environmental sustainability will determine how rapidly genomic medicines can be scaled to meet global demand.
Feedstock purity for nucleosides influences the purity of final drug substance. Terminal α-anomer prevents full chain extension. Trace palladium(II) cross coupling catalyst is reductively activated, leading to oxidative depurination. Endogenous fluorescent etheno-adducts co-elute with intact oligonucleotides, rendering them inseparable by purity analysis. Excess endotoxin in nucleotide triphosphate causes spurious positive Toll-like receptor activation assays in in-vitro immune studies, and over-design of immune evasive base modifications. Heavy-metal contamination >1 ppm can be mis-incorporated as 8-oxo-adenosine, causing collapse of duplex stability with promutagenic lesions. In contrast, using crystalline, isotopically labelled adenosine allows for use of mass-spec detection to track disposition, and greatly simplifies impurity profiling for regulatory submissions. In summary, high-purity adenosine is not just a quality luxury, but the kinetic hinge that swings an oligonucleotide program between late-stage GMP manufacture or a return to synthesis without specification drift.
Adenosine and deoxyadenosine have an identical bicyclic purine core that offers three hydrogen-bond donors and one acceptor, so both molecules can present Watson–Crick and Hoogsteen pairing geometries. The only structural difference is the substituent at C-2 of the sugar: the hydroxyl group of adenosine fixes the ribose in C3'-endo (north) pucker, a prerequisite for RNA helices; in deoxyadenosine, a proton pushes the pucker equilibrium towards C2'-endo (south) and confers the chemical stability needed for DNA storage. The atomic-scale switch is applied at the monomer level and translated into duplex rigidity, nuclease resistance and innate-sensor engagement without any impact on the amidite coupling cycle.
Table 1 Atomic-level distinctions and therapeutic read-outs
| Structural element | Adenosine (A) | Deoxyadenosine (dA) | Functional consequence |
| Sugar C-2' | -OH | -H | RNA vs DNA pucker |
| Base pairing | WC + Hoogsteen | WC only | Duplex flexibility |
| Innate sensor | RIG-I substrate | Minimal | Immunogenicity dial |
| Common amidite | 2'-OMe-A | dA | Platform selection |
The purine ring is a planar, electron-rich system containing five nitrogens and four carbons that can act as chemical handles: N1, N3, N7, C2, C6, and the exocyclic 6-amino group. The glycosylation site N9 is the connection point of the base to ribose (adenosine) or 2-deoxyribose (deoxyadenosine) via a β-N-glycosidic bond. Orthogonal handles on the base itself include N6 for methylation, C8 for azide/alkyne click chemistry and N7 for metal chelation without interfering with Watson-Crick base pairing. Since the purine ring is aromatic, it has a UV-absorbance maximum at 260 nm, which makes real-time trityl monitoring of solid-phase synthesis and quantitative HPLC release testing possible.
Ribose adenosine favors C3'-endo pucker which constricts the major groove and augments helical twist, two properties required for both siRNA seed-region flexibility and ribosomal decoding; the 2'-hydroxyl is also an internal nucleophile that speeds base-catalyzed cleavage, requiring 2'-OMe or 2'-F protection for therapeutic oligos. Deoxyribose biases the equilibrium toward C2'-endo, which expands the major groove and results in the B-form geometry that recruits RNase H when integrated into gapmer cores. These conformational disparities are encoded at the monomer level, allowing developers to switch between the states of catalytic cleavage (DNA) and high-affinity silencing (RNA) simply by choosing the proper sugar amidite, without the need to re-engineer the solid-phase cycle.
Orthogonally modifiable nucleobases provide handles for post-synthetic functionalization. The N6 exocyclic amino group is labilely protected with benzoyl chloride to avoid transamidation during activation of the phosphoramidite and the C-8 position is tolerant to bromination or fluorination to improve electron-withdrawal and avoidance of RIG-I recognition. The 5'-hydroxyl is derivatized with dimethoxytrityl (DMT) ether to permit directional elongation, and the 3'-hydroxyl is activated with bis(diisopropylamino)chlorophosphine to form the amidite that couples under tetrazole catalysis. As these orthogonal handles can be appended without disrupting the β-anomeric configuration, the same nucleoside can be repeatedly elongated to afford stereopure oligonucleotides whose pharmacokinetics are pre-encoded at the monomer level. This provides a chemically robust scaffold for therapeutic RNA and DNA alike.
Adenosine derivatives are the purine alphabet of programmable gene drugs and include native ribosides (encoding Watson–Crick accuracy) through to completely synthetic amidites (featuring 2'-fluoro, N6-lipid or 8-azaguanine modifications); the choice of derivative will decide whether the final oligonucleotide will be a silencer, translator or folder into a high-affinity aptamer without rewriting the synthetic chemistry.
Native adenosine has a ribose cis-diol that biases it to C3'-endo puckering, thus pre-organizing the A-form helices required for RISC loading and ribosomal decoding; deoxyadenosine does not have the 2'-OH group, which biases it to C2'-endo flexibility, which in turn opens the major groove and presents an optimal RNase-H recognition floor within gapmer cores. Crystallization can cleanly separate the isomers, resulting in β-anomeric solids that are soluble in anhydrous acetonitrile and can be coupled at >98 % stepwise efficiency after phosphitylation. The absence of protecting groups simplifies early-stage scouting, although the free N6-amino must be temporarily benzoylated to prevent acyl migration during oxidation. Native adenosines are thus the least-cost starting point for kilogram-scale solid-phase campaigns, provided that mild depurination controls are built into the cycle protocol.
5'-O-Dimethoxytrityl-N6-benzoyl-adenosine is the standard phosphoramidite precursor: the acid labile DMT group masks the 5'-hydroxyl for repetitive detritylation, while the base-labile benzoyl group protects the exocyclic amine from transamidation during oxidation. 2'-O-TBDMS-adenosine bears a fluoride labile silyl ether, thus facilitating chimeric RNA/DNA strands with ribo segments providing A-form geometry and deoxy segments inviting catalytic cleavage. 2'-O-MOE-protected adenosine features a lipophilic ethyl ether that increases nuclease resistance without increasing steric bulk, as needed in gapmer wings where affinity must increase while RNase-H recruitment is preserved. These orthogonally protected derivatives are purified by crystallization, giving single-diastereomer solids that can be stored for years under argon, allowing just-in-time dissolution to anhydrous acetonitrile for automation amenable coupling cycles.
Replacement of the imino proton of adenosine with a methyl in 8-Aza-7-deaza-adenosine extinguishes TLR7 activation due to perturbation of the Hoogsteen face, while maintaining argonaute compatibility. This may be employed in liver-targeted siRNA, where knockdown of cytokines that modulate innate immune activity may be desired. Increased lipophilicity due to addition of two methyl groups in N6-Dimethyl-adenosine shifts the interaction of oligonucleotides to favor albumin binding and retard renal clearance without geometrically affecting base-pairing. Replacement of O6 with sulfur in 2-Amino-6-thio-adenosine to increase Hoogsteen hydrogen bonding capability and duplex melting temperature allows for employment in gapmer wings to better recruit RNase-H. Locking the glycosidic bond in syn conformation with 8-Bromo-adenosine results in increased stability of antiparallel G-quadruplexes with nanomolar affinity for heparin-binding growth factors. Modifications can be added postsynthetically or by using pre-formed, modified amidites, and an oligonucleotide can thus possess both immunosilent and high-affinity elements without rewriting the genomic sentence.
The last step of the synthesis is the attachment of a 2-cyanoethyl-N,N-diisopropyl-phosphoramidite to the 3'-hydroxyl group, which is carried out in anhydrous conditions via phosphitylation. The reaction does not racemize because the vicinal 2'-substituent rigidifies the furanose ring, so that only the β-anomer is produced, which is needed for Watson–Crick base-pairing. The phosphoramidite is crystallized from acetonitrile/hexane, to both remove excess reagent and remove trace water in one filtration. The cyanoethyl group is a temporary phosphate protecting group that auto-cleaves during mild basic oxidation. The group removes the need for an additional deprotection step. Bottled under argon, the solid amidite is stable on the shelf for years. It can be dissolved just-in-time from the solid into anhydrous acetonitrile and used for automated coupling cycles, to produce oligonucleotide drug material ranging in scale from milligram to multi-kilogram without re-optimization.
Adenosine derivatives are the energy currency of the siRNA, ASO and mRNA scaffolds: the bicyclic base enhances base-stacking and the sugar is accessible at the monomer stage to be locked, fluorinated or N-alkylated to pre-program nuclease resistance, duplex stability and evasion of innate-sensors without disrupting the amidite coupling cycle—permitting the same automated synthesiser to produce gene-silencing siRNA, splice-switching ASO or self-amplifying mRNA whose PK is pre-defined at the adenosine level.
siRNA can have 2'-fluoro-adenosine in the seed region (positions 2–8) to stiffen the guide strand against serum nucleases and to retain A-form geometry for loading into argonaute 2; N6-methyl-adenosine at position 14 can prime RISC maturation without activating TLR7. Passenger strands are typically modified with 2'-O-methyl-adenosine to prevent off-target slicing and 8-aza-7-deaza-adenosine to quench any residual immunogenicity. These changes are often in a mosaic pattern, fluoro for catalysis, methoxy for nuclease resistance, aza for transcriptional silence, resulting in duplexes that can silence transthyretin or PCSK9 mRNA for months after subcutaneous injection. Since adenosine edits don't change the Watson–Crick edge geometry, potency is gained without re-engineering the targeting algorithm, so the purine is the tunable heart of RNAi therapeutics.
Gapmer ASOs have a deoxyadenosine scaffold at their core with internal DNA-like stretches to guide RNase H1 to the huntingtin or SMN2 RNA for cleavage; the 2'-MOE-adenosine wings on the ends of gapmers provide higher binding affinity and exonuclease resistance without disrupting catalytic geometry. Steric-block splice-switchers have a uniform 2'-O-methoxyethyl-adenosine (2'-MOE-A) composition across their full length, which represses SMN2 exon 7 skipping by occluding the splice silencer but does not induce RNA degradation. Phosphorothioate adenosine diesters have an asymmetric sulfur atom that can increase tissue half-life by binding to albumin but have a potential for off-target pro-inflammatory effects; this potential can be abrogated by the incorporation of N6-methyl-8-oxo-adenosine, which maintains sulfur-induced stability but suppresses TLR9 activation.
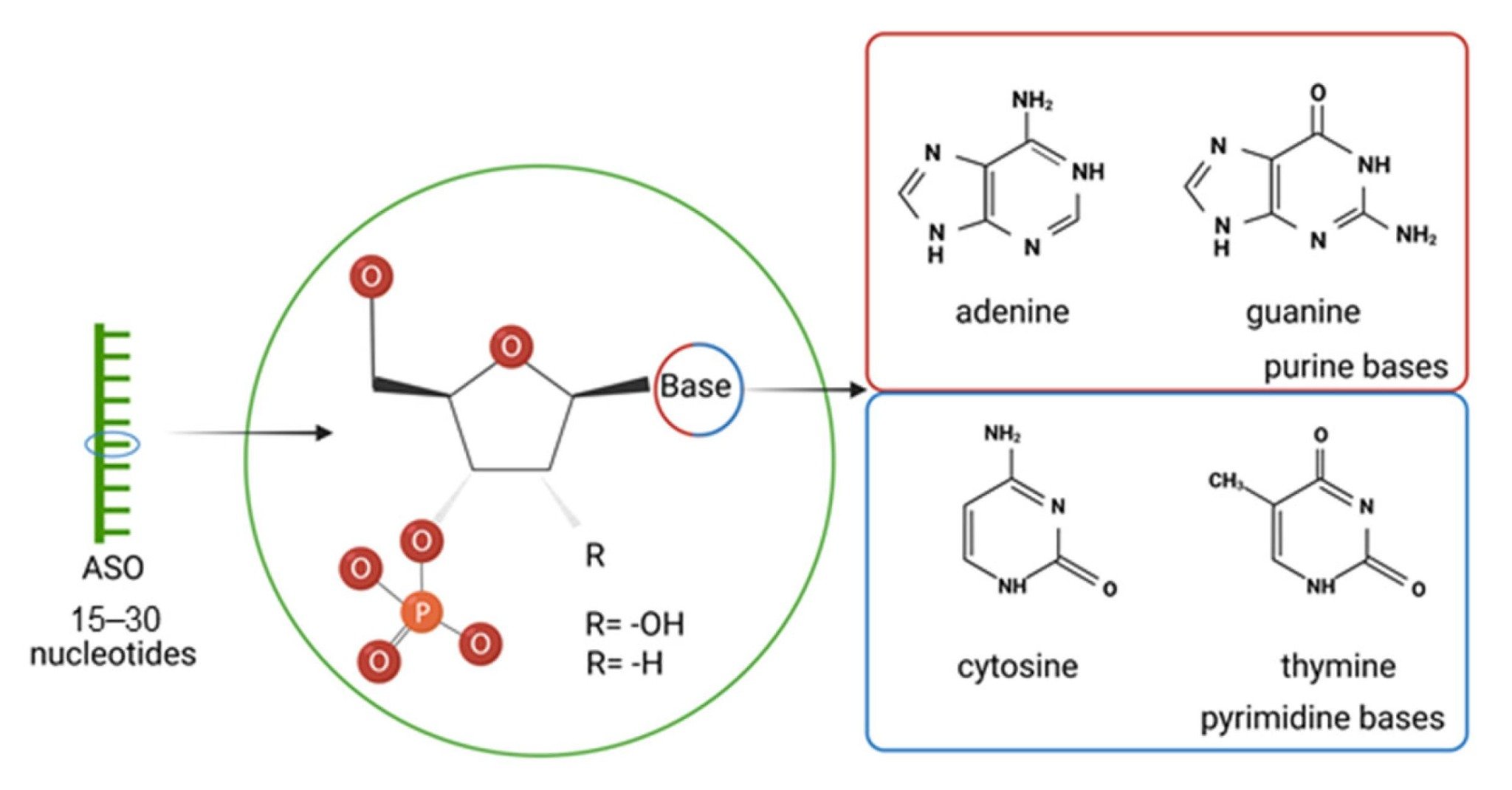 Fig. 2 Structure of antisense oligonucleotides (ASOs).2,5
Fig. 2 Structure of antisense oligonucleotides (ASOs).2,5
mRNA vaccines: Full substitution with N6-methyl-pseudoadenosine plus 5-methoxy-uridine abolishes PKR and RIG-I signalling to allow self-amplifying replicons to produce monoclonal antibodies with microgram-per-millilitre titres in serum. 2-amino-6-thio-adenosine triphosphate as building block in circular RNA precursors to install reversible thiol cross-links for ribosomal shunting before reductive cleavage. Guide RNAs for CRISPR include 2'-fluoro-N6-dimethyl-adenosine at the 5'-end to improve Cas binding while the 3'-end is native to maintain authentic termination. Adenosine derivatives are thus programmable chemical pixels that toggle translational output, circularisation efficiency or gene-editing fidelity on demand to make the purine the universal adaptor between sequence and therapeutic phenotype.
The need for high-purity adenosine derivatives, for example, is absolute for the production of therapeutic quality oligonucleotides. Impurities such as trace α-anomers or metal ions and water, which cannot be removed in the chain elongation step, terminate synthesis, initiate formation of immunogenic side-products and alter the PK profile. One impurity in the monomer that goes undetected can lead to the loss of a kilogram of API and months of regulatory filing dates.
The residual α-anomer of adenosine phosphoramidite functions as an intrinsically unreactive chain terminator. The unnatural glycosidic geometry inhibits tetrazole-mediated phosphitylation, which results in reduction of the stepwise yield to<90 from="">98 % and truncated 20-mer contaminants co-eluting with full-length product in ion-pair HPLC. Trace water hydrolyses the reactive P(III) amidite to H-phosphonate, which consumes activating tetrazole and leads to diester linkages that collapse into abasic sites upon basic deprotection. Free adenine base created through acid-catalysed depurination competes with the amidite during coupling, incorporation of base-less ribose units that destabilize the duplex and lower the melting temperature.
Metal-ion impurities (Pd, Cu) from previous Suzuki or Sonogashira cross-couplings catalyze oxidative depurination during extended ammonia deprotection, transforming adenosine into etheno-adducts that fluoresce at 420 nm and copurify with the product, thereby confounding absorbance-based quantification. Excess solvent (dichloromethane) can alkylate N1 to give quaternary adeninium salts that skew the glycosidic torsion toward syn, destabilizing G-quadruplex aptamers that are designed to be parallel topology. Water from packaging hydrolyses the cyanoethyl protecting group prematurely, forming phosphodiester linkages that can be cleaved by serum nucleases, reducing in-vivo half-life. In this way, monomer-level impurity signatures are preserved in the finished oligonucleotide as permanent structural mutations, obliterating sequence fidelity and therapeutic index both.
Detectable levels of Palladium (>10 ppm) cause side cross-linking of adenosine C8 and guanosine N2 under oxidative work-up to create fluorescent by-products that cause false genotoxicity warnings. Chlorinated solvents that are not distilled off (>500 ppm) may react to form adenine-chlorohydrins under basic deprotection. This mutagenic lesion is not typically detected by MS. Residual water (>200 ppm) hydrolyses the phosphoramidite P(III) centre to H-phosphonate diesters, which during ion-pair HPLC fragmentation causes ghost peaks that are mistaken for failure sequences. These impurities drive higher analytical load, force expensive re-purification steps and can cause regulatory challenges in the specification rationalization. Therefore, high purity adenosine is not just a quality extravagance. It is the kinetic fulcrum between whether the oligonucleotide programme will proceed smoothly into late stage GMP or must face re-synthesis and/or specification drift.
Setting robust quality standards for adenosine derivatives is critical to their suitability for use in research and clinical applications. Quality standards encompass acceptable ranges for purity, structural integrity, and residual impurities, all of which collectively affect the reliability and reproducibility of oligonucleotide synthesis. The quality of the adenosine used can have a significant impact on the coupling efficiency, sequence fidelity, and overall performance of the final product, whether it is siRNA, mRNA, or antisense oligonucleotides. Quality control measures must be in place, ranging from analytical characterization to batch consistency and full GMP documentation, especially when scaling up to therapeutic-grade production.
The purity specifications of building blocks intended for the manufacture of research grade oligonucleotides are generally considered to be ≥98 % by HPLC-UV, residual solvents restricted to Class 3, and moisture content of<0.5 %. By contrast, GMP-grade building block specifications are typically ≥99 % chemical purity, ≤0.1 % free base, and residual elemental impurities (especially Pd, Cu) are tightly controlled below 1 ppm because of their catalytic impact on depurination. Anomeric purity should also be confirmed to be ≥99 % β-configuration in order to avoid chain termination during solid-phase synthesis. Endotoxin levels of nucleotide-grade adenosine used for enzymatic RNA synthesis should be <0.25 EU/mg and bioburden should be ≤10 CFU/g.
Structural identity is demonstrated by 1H-NMR and 13C-NMR to confirm the β-glycosidic linkage and to rule out the presence of regioisomers. HPLC-UV (260 nm) analysis is used to determine the chemical purity. Gradient HPLC methods are usually sufficient to resolve 2'-OMe, 2'-F and native adenosine variants. MS (ESI-QTOF) provides confirmation of molecular weight and the presence of low-level adducts (e.g., 8-oxo or N6-etheno, etc.). 31P-NMR is also used in the case of phosphoramidite precursors to confirm integration of the phosphate and absence of the H-phosphonate side-product. Residual solvents are determined by GC-HS analysis and ICP-MS is used to screen for trace metals. As a minimum, all of these orthogonal methods are applied to every batch to provide assurance that the material is structurally authentic, chemically pure and devoid of process-related impurities that might be expected to interfere with further synthesis or activity in biological assays.
ICH Q7 and Q11 require at least five consecutive batches to be assessed in order to show batch-to-batch consistency, which is often set with acceptance criteria based on ±3 SD from the mean value. The CoA's of individual batches must provide all analytical data with attached full NMR spectra, HPLC chromatograms, and MS profiles. Reference standards need to be qualified against either USP or in-house primary standards and expiry and retest intervals must be documented. Any changes in anomeric ratio, moisture content or elemental impurity profile needs to be justified after further investigation. Stability studies should be carried out and reported that adenosine derivatives maintain stability in purity and functionality over the stated shelf-life when stored in inert atmosphere at −20 °C. All of this documentation is required for regulatory submissions as well as providing full traceability through the supply chain.
Scale-up of the synthesis of adenosine derivatives requires careful consideration of route planning and quality control: the more complex the target molecule, the greater the need for a synthetic route that is robust and ensures chemical consistency between batches. Manufacturers compare different routes for overall yield, cost, raw material availability, and suitability for implementation in current good manufacturing practice (cGMP) settings. Scaling from lab-scale synthesis to pilot or commercial scale can expose challenges such as heat transfer, impurity management, and equipment speciality. Continuous-flow chemistry and real-time analytics are being implemented for consistent product quality and risk mitigation during scale-up.
Table 2 Scale-up considerations for adenosine derivatives
| Parameter | Lab-scale | Pilot-scale | Commercial-scale | Control strategy |
| Solvent use | High | Moderate | Minimized | Solvent recycling |
| Reactor type | Batch | Fed-batch | Continuous-flow | Inline monitoring |
| Impurity profile | Simple | Complex | Defined | Real-time analytics |
| Quality control | Offline | Hybrid | Inline | PAT integration |
Route selection is the first and most fundamental scale-up decision. Chemists will compare synthetic routes based on the number of steps, the overall yield, the availability of starting materials and their green credentials. Routes to adenosine derivatives include Vorbrüggen glycosylation and separation via selective crystallization (to control anomeric purity) or enzymatic transglycosylation if a greener solution is preferred. Continuous-flow platforms are increasingly popular over traditional batch reactors since they enable improved heat transfer, reduced reaction time and decreased solvent volumes. Inline analytics such as FTIR or Raman spectroscopy provide real-time reaction monitoring and help to ensure that critical quality attributes (anomeric ratio, base integrity, etc.) are consistent across scales.
Scale-up of the adenosine derivatives presents certain problems which were not encountered on laboratory scale. Heat transfer becomes an issue during the exothermic glycosylation steps, making the reaction reactors and temperature control critical. Stereochemical integrity must be maintained during crystallization, which needs specific cooling profiles and seed-loading methodology. Impurity levels are more significant and require robust purge strategies (reslurry or carbon treatment). Drying and storage of some intermediates is problematic due to their hygroscopic nature. Drying can also be an issue in some cases. The need for anhydrous conditions adds to the complexity of the process. These issues are overcome by the use of continuous-flow synthesis, stereoselective crystallization and real-time analytical technologies to achieve batch-to-batch reproducibility.
Supply chain robustness is also an important aspect of clinical supply. Sourcing of starting materials with necessary high levels of purity, for example adenine, ribose or deoxyribose, and protecting reagents, is assured by dual sourcing of key materials and having long-term contracts in place to guard against shortfalls due to geopolitical or market fluctuations. Overall lead times are reduced by coupling continuous-flow synthesis to inline purification and quality control, so minimizing the need for offline testing. Plant design has been modularized to allow rapid scale up or tech-transfer between facilities, and disposable flow paths further limit cross-contamination risk and cleaning validation requirements. This allows the same synthetic route to be performed without regulatory delay across multiple sites.
We offer a specialized portfolio of high-purity adenosine (A) derivatives designed to meet the demanding requirements of oligonucleotide drug development. Our products and services support RNA- and DNA-based therapeutic platforms by combining reliable raw material quality with technical expertise across synthesis, scale-up, and manufacturing.
Our portfolio includes a full range of adenosine and deoxyadenosine derivatives suitable for RNA and DNA oligonucleotide synthesis. These materials are manufactured to stringent specifications to ensure high chemical purity, controlled impurity profiles, and low moisture content, all of which are critical for reproducible oligonucleotide synthesis. Both native and synthesis-ready adenosine derivatives are available to support downstream conversion into phosphoramidites or other activated intermediates. Consistent batch-to-batch performance helps ensure predictable coupling efficiency and reliable final product quality across development programs.
To support modern oligonucleotide design, we supply modified and protected adenosine derivatives optimized for solid-phase synthesis. This includes adenosine monomers with base and sugar protection as well as clinically relevant modifications designed to enhance stability and biological performance. Our protected A derivatives are engineered to provide clean reaction profiles, high coupling efficiency, and efficient deprotection, minimizing side reactions and sequence-related impurities. These characteristics are essential for maintaining sequence integrity and achieving high yields, particularly in complex or highly modified oligonucleotide sequences.
For programs with specific or novel requirements, we offer custom adenosine derivative development and process optimization services. Our technical team supports route selection, protecting group strategy design, and impurity control to ensure that custom A derivatives are both chemically robust and scalable. By working closely with customers, we help translate early-stage molecular designs into manufacturable raw materials suitable for long-term development and commercial production. This collaborative approach reduces technical risk and supports efficient progression from discovery to clinical manufacturing.
We provide flexible supply options to support adenosine derivatives across all stages of development. From small quantities for research and feasibility studies to larger volumes for preclinical and GMP manufacturing, our supply model is designed to scale with your program. Materials are supported by clear specifications, traceability, and appropriate documentation, enabling smooth transitions between development stages while meeting regulatory and quality expectations. This flexibility helps ensure continuity and reliability as oligonucleotide programs advance.
If you are looking for high-purity adenosine or deoxyadenosine derivatives supported by experienced technical expertise, we are ready to assist. Contact us today to request technical information, samples, or quotations, or to discuss custom adenosine derivative development and scalable supply solutions for your oligonucleotide program.
References
Adenosine is a core purine base used extensively in RNA and DNA therapeutics.
Typically ≥98–99% purity with controlled impurities and moisture.
Impurities reduce coupling efficiency and increase failure sequences.
Yes, especially in RNA therapeutics to improve stability and performance.
Yes, GMP-ready adenosine derivatives are available for clinical manufacturing.

