Chemical capping agents, which are used instead of the multi-step enzymatic reactions described above, involve the addition of a single reagent to the IVT mixture. This reagent provides a pre-synthesized cap analogue that is recognized and used as a primer by the RNA polymerase. Use of this method instead of the enzyme reaction steps above, which require incubation with guanylyl-transferase and methyl-transferase, reduces the time required for capping and results in a uniform 5'-terminal structure that is fully translational competent and is not subject to proprietary licensing issues.
Chemical capping refers to the co-transcriptional addition of a chemically synthesized cap analogue, usually an m7GpppG with a 3'-O-methyl, 3'-deoxy, or other blocker modification so that the nascent transcript emerges from the polymerase with the endogenous 5'→5' triphosphate bridge already in place; since the analogue is the initiating nucleotide, the mRNA is protected from exonucleases, is primed for efficient ribosome binding, and can be generated in the lab under normal conditions without additional enzymes or buffer exchanges.
There are two main methods for adding the 5' cap: Enzymatic post-transcriptional capping involves the use of vaccinia or faustovirus capping enzyme with methyl donors to transform the triphosphate end into Cap 0 or Cap 1 while chemical capping involves simply adding a cap analogue to the IVT reaction so that the polymerase itself adds the cap in the proper orientation during chain initiation; the latter method does not require additional incubation steps, leads to less contamination with potentially immunogenic triphosphate species and can be easily scaled linearly with reaction volume, and is thus the preferred method for both high-throughput screening and early-stage therapeutic campaigns.
Stepwise enzymatic capping requires at least two reaction steps with separate buffers, and specific inputs of GTP and S-adenosyl-methionine. Also, both enzymes need to be active at the same temperature which is not always the case. The additional manipulations expose the RNA to increased RNase risk and extended duration of the procedure which is more costly. Furthermore, if the reaction is incomplete, immunostimulatory triphosphate ends can remain that may activate innate immune sensors and reduce translational efficiency. This is especially a problem when a high degree of batch-to-batch reproducibility is necessary for the application and when working with limited resources.
Chemical capping is becoming more popular because it streamlines capping and transcription into a single reaction vessel, eliminates the need for expensive enzymes and cofactors, and provides a reproducibly high fraction of properly oriented caps; the resulting mRNA has lower innate immunogenicity, better translational efficiency, and is compatible with both lithium-chloride precipitation and chromatographic purification, all properties that are well suited to the speed, cost-containment, and intellectual-property freedom requirements of contemporary RNA prototyping and decentralized manufacturing efforts.
Table 1 Comparison of capping strategies
| Attribute | Enzymatic post-IVT | Chemical co-transcriptional | Strategic implication |
| Unit operations | 3 enzymes, 2 purifications | 1 reagent, 1 pot | Faster release, lower COGS |
| Cap architecture flexibility | Fixed enzyme set | Swap reagent bottle | Rapid iteration |
| Scalability ceiling | Enzyme production | Organic synthesis | Metric-tonne capability |
| Regulatory file | Enzyme master file | Reagent DMF | Simpler CMC pathway |
| Orientation control | None (Cap 0) | ARCA/trinucleotide | >90 % forward yield |
Chemical capping takes advantage of the T7, SP6 or T3 polymerase property of beginning RNA synthesis with a synthetic cap dinucleotide in place of GTP; an excess of a methylated cap analogue over GTP ensures that virtually every transcript is initiated with the correct 5'→5' linkage, so the mRNA leaves the same reaction vessel already protected against exonucleases and ready for ribosome binding with no need for post-synthetic enzymatic modification.
During elongation, the polymerase scans the template for the first encoded nucleoside triphosphate, so when a cap analogue is present at higher concentration than GTP, the enzyme accepts the dinucleotide as a pseudo-primer and extends the RNA chain from its 3'-OH, thereby fusing the cap directly to the +1 position, while the triphosphate bridge is formed in the same catalytic cycle, ensuring that capping and transcription are completed simultaneously inside a single closed reactor.
Cap analogues serve as an initiator and protecting group: the N7-methylguanosine moiety serves as a blocker for 5'-exonucleases, the 5'-5' triphosphate as the docking site for eIF4E, and chemical modifications like 3'-O-methyl or 2'-O-methyl suppress reverse incorporation or endogenous sensing; by modulating these substituents, one can balance incorporation efficiency, immune evasion and translational potency without having to change the DNA template or the polymerase.
The conditions required for optimal chemical capping are somewhat contradictory. Mg2+ must be high enough to be close to the polymerase optimum to avoid reducing the elongation rate; but low enough to keep pH below 8, to avoid alkaline hydrolysis. GTP must be low, so that the cap analogue outcompetes it for the initiation site; but the total concentration of NTPs should be high, to avoid global termination. Gentle mixing and low salt avoid local depletion of the cap. Taken together, these ensure that capping efficiency, transcript length and yield remain reproducible when scaling up the reaction.
Chemical and enzymatic capping are different approaches for 5'-end mRNA cap formation: In chemical capping, chemically synthesized cap analogs are used for co-transcriptional mRNA capping. The enzymatic capping is performed post-transcriptionally with a cascade of enzymes encoded by the virus. The two approaches differ in their translation efficiency, immunogenicity, processing steps, associated costs, and GMP-compliance requirements. The main advantage of the chemical capping approach is the control over the orientation of the cap and the one-pot process. Drawbacks of chemical capping are the lower overall yield and competition of cap analogs with GTP. The main advantage of the enzymatic approach is the near-quantitative capping and formation of the native Cap-1 methylation. The additional steps in enzymatic capping lead to additional unit operations, enzyme impurities, and higher requirements for suppliers' management. In this chapter, we will compare these two methods in terms of efficiency, scalability, cost, and downstream processing.
Chemical capping efficiency is dictated by competition between the cap analog and GTP at the polymerase active site. Even under optimised cap: GTP ratios (usually 3–5:1) forward incorporation can approach >80 % for ARCA type dinucleotides and >90 % for trinucleotide CleanCap reagents, but overall RNA yield is often much lower because the bulky analog inhibits elongation . Enzymatic post-IVT capping, by contrast, occurs on pre-formed RNA and routinely achieves >95 % completion, independent of transcript length or sequence context, because the vaccinia capping enzyme recognises the diphosphorylated 5' end with high specificity . The trade-off is therefore between a high-yield, lower-potency chemical route and a lower-yield, higher-potency enzymatic route, with the final choice dictated by whether overall RNA mass or functional RNA (correctly capped) is the critical quality attribute.
Chemical capping requires no change to the overall IVT workflow—only a scaled-up reagent loading corresponding to the increased cap analog concentration in the IVT mix. There are no added reactors, buffer exchanges, or enzyme qualification steps. The reagents are crystalline solids, which can be stored at metric-tonne scale and dosed in exactly the same way as any other nucleotide or reagent. This makes it inherently friendly to single-use, continuous-flow, or microfluidic platforms. In contrast, enzymatic capping requires a second, temperature-controlled reaction vessel, the addition of three recombinant enzymes, careful S-adenosyl-methionine management, and a final purification step to remove proteinaceous impurities and reaction by-products. Each of these extra unit operations requires added hold times, adds to bioburden risk, and necessitates release testing for enzyme activity and endotoxin. All of these become disproportionately expensive when batch size increases beyond the gram scale.
On a cost-per-functional-transcript basis, chemical capping is usually lower for research and early-clinical scales, since the reagent premium is amortized by the savings in enzyme purchase, QC and labor. ARCA is ~15 % of raw-material cost, but obviates a two-hour enzymatic incubation and a second purification column, so it saves operator time and facility overhead as well. Enzymatic capping becomes competitive only when ultra-high capping efficiency (>98 %) is required, or if the desired cap structure (e.g., Cap 2) is not yet available as a stable analog. For personal neo-antigen libraries, where scores of constructs are run in parallel, the speed and simplicity of chemical capping outweighs the incremental reagent cost, whereas for billion-dose vaccines, the enzyme cost is amortized and the higher yield of enzymatic capping can dominate the economic model.
Chemical capping also streamlines downstream purification, as the only novel impurity is cap analog residuals, a low-molecular-weight species easily removed by tangential-flow filtration or anion-exchange chromatography. Enzymatic capping in contrast adds proteinaceous impurities (capping enzymes, BSA stabilizers, endotoxin) that must be cleared with protease digestion/heat inactivation or additional chromatography steps, each at a cost and analytical burden. In addition, residual S-adenosyl-homocysteine can inhibit downstream methyltransferases if the mRNA is subject to further modification (e.g., internal m6A), whereas no such by-product is formed with chemical capping. The net result is typically one less purification step and half the QC assays (no enzyme activity, no residual SAM) with chemical routes, translating into faster release timelines and lower COGS for clinical batches.
Chemical capping reagents are small-molecule mimics of the enzymatic cascade, the initiating nucleotide of which is substituted during IVT to effect an orientation-controlled, methylation-encoded cap in one closed-tube step. The toolbox ranges from basic dinucleotides to advanced trinucleotides that encode Cap-1 or Cap-2 structures, each engineered for a desired balance of translation efficiency, immune-evasion, and process simplicity.
The simplest reagent is m⁷GpppG, which generates Cap-0 but is subject to reverse incorporation due to T7 polymerase being able to start from either hydroxyl of the guanosine ribose. Anti-reverse cap analogs (ARCA) address this by methylating or deoxygenating the 3'-OH, ensuring initiation is only from the 2'-OH and increasing the fraction of properly oriented caps to over 80 %. Cap-1 chemistry can be achieved without a post-enzymatic step by using trinucleotide analogs such as m⁷GpppAmpG: the 2'-O-methyl group on the adenosine residue is co-transcriptionally installed, generating transcripts that already bear the "self-RNA" mark recognized by eIF4E and that block IFIT1 binding. These systems work with typical IVT buffers and only require a modest excess of cap over GTP. These therefore are the default systems used for the majority of vaccine and protein-replacement workflows.
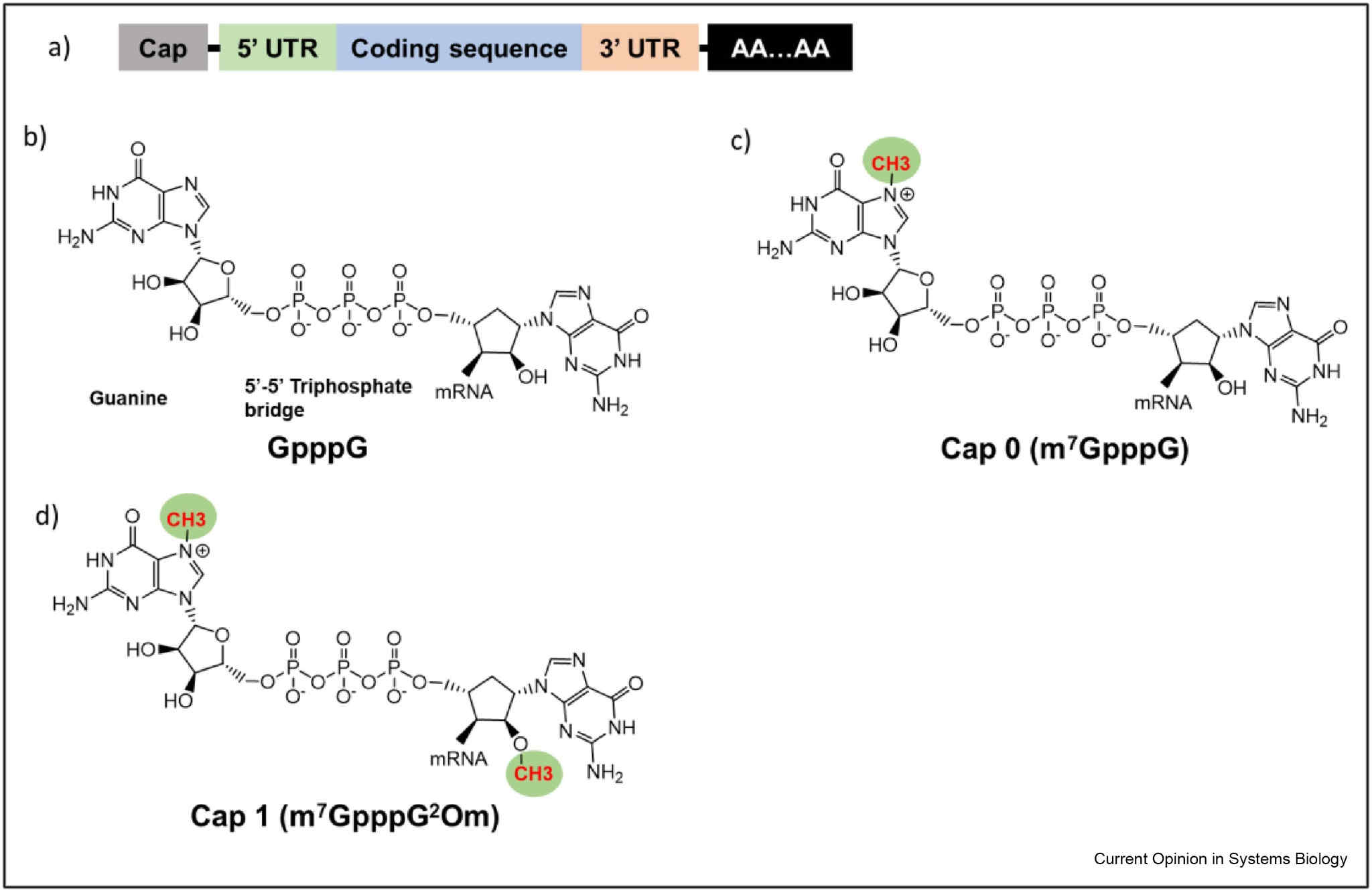 Schematics of a) an intact RNA molecule, b) G cap, c) cap 0, and d) cap 1.1,5
Schematics of a) an intact RNA molecule, b) G cap, c) cap 0, and d) cap 1.1,5
ARCA reagents are dinucleotide analogs in which the 3'-OH of the guanosine moiety is blocked, so as to prevent reverse initiation, while retaining the natural 5'-5' triphosphate bridge. The 3'-modification is generally introduced through 3'-O-methyl or 3'-deoxy chemistry, so the final reagent is a diastereomeric pair that can be resolved chromatographically into pure "forward" material. Extended-phosphate variants (tetra- or pentaphosphate) are often used to further increase intrinsic affinity for eIF4E without changing the orientation control element. ARCA-capped mRNA demonstrates two- to three-fold greater translation efficiency than the standard m7GpppG, but is still a Cap-0 structure, so a subsequent 2'-O-methyltransferase step is needed in order to generate full Cap-1 immune evasion if desired. The reagents themselves are crystalline, shelf-stable, and readily soluble in standard IVT buffers, and so can be used as a drop-in upgrade for research-scale or personalised therapies where rapid, low-cost synthesis is desired more than full Cap-1 stealth.
Trinucleotide cap analogs (m7GpppAmpG, m7GpppCmpG, etc.) contain both N7-methylguanosine and the 2'-O-methylated first nucleotide within the same molecule, thereby providing Cap-1 co-transcriptionally and without the need for downstream enzymatic processing. The first nucleotide is pre-methylated which prohibits the polymerase from adding it in the reverse orientation, thereby producing >90 % forward caps that do not require optimization of the cap to GTP ratio. The trinucleotide also forces an AG, CG or UG start codon, which reduces upstream AUG artefacts that can generate truncated peptides. These systems are also compatible with high-yield T7 polymerase variants and can be lyophilized, making them the system of choice for billion-dose vaccine campaigns and clinical-grade therapeutics, where consistency, speed, and regulatory familiarity of the reagent offsets its higher unit cost.
All chemical capping reagents must be recognized as primers by T7 RNA polymerase, which has limited the structural complexity of these reagents. However, this issue has largely been addressed through iterative SAR studies, where the wild-type T7 polymerase accommodates m⁷GpppG and ARCA analogs with only a modest loss in elongation rate, while high-fidelity variants can incorporate 2'-O-methylated trinucleotides without significant loss in yield. Other important parameters include the Mg²⁺ concentration (5–10 mM), the pH (7.5–8.0), and the solubility of the analog. Insoluble or very bulky caps can either precipitate or function as chain terminators. Real-time monitoring by RP-HPLC has been used to track forward/reverse cap ratios across scales to ensure that the polymerase compatibility holds from 50 µL screening reactions to 10 L single-use bioreactors.
Chemical capping reagents are often described as the missing element in elevating et al. transcription from a science project to a scalable, clinical-ready manufacturing platform. By incorporating the desired cap (Cap 0, Cap 1 or Cap 2) directly into the initiating nucleotide, chemical capping reagents circumvent post-synthetic enzymatic steps, mitigate open-handling bioburden risk, and streamline the entire workflow for alignment with single-use, closed-system expectations. The practical applications are therefore as broad as the development spectrum: spanning the high-throughput screening plates that require only milligram quantities, to kilogram-scale bioreactors that supply global vaccine campaigns, to personalized neo-antigen libraries where each patient's therapy is synthesized within hours of tumor sequencing.
Chemical capping reagents are metered as crystalline powders that dissolve into the IVT buffer, eliminating the cold-chain and endotoxin limitations of enzyme post-capping. Trinucleotide CleanCap-type analogs are formulated in multi-kilogram bags of diastereomerically pure material, and as such can support the production of capped mRNA with >90 % forward orientation from a single 10 000 L single-use bioreactor, with no post-IVT polishing. As the reaction is stoichiometric, the cap-to-GTP ratio can be monitored and tuned in real time using inline Raman spectroscopy, which allows the capping efficiency to be maintained at the desired setpoint, even if the polymerase activity drifts (for example, due to fluctuations in template GC-content or magnesium). The lack of an enzyme master file also streamlines technology transfer between CMOs, and regional supply hubs can maintain an inventory of the reagent using standard nucleotide storage conditions, thereby insulating the manufacturing timelines from the historically months-long lead times for enzymes.
For preventive vaccines, chemically capped RNA provides the Cap-1 self-epitope required to prevent IFIT1-mediated translation repression without sacrificing the speed required for pandemic-response scale. Reagents are compatible with lipid-nanoparticle encapsulation and lyophilization, allowing the same bulk RNA to be formulated into intramuscular injectables, intranasal sprays, or dermal microneedle patches without loss of the cap.
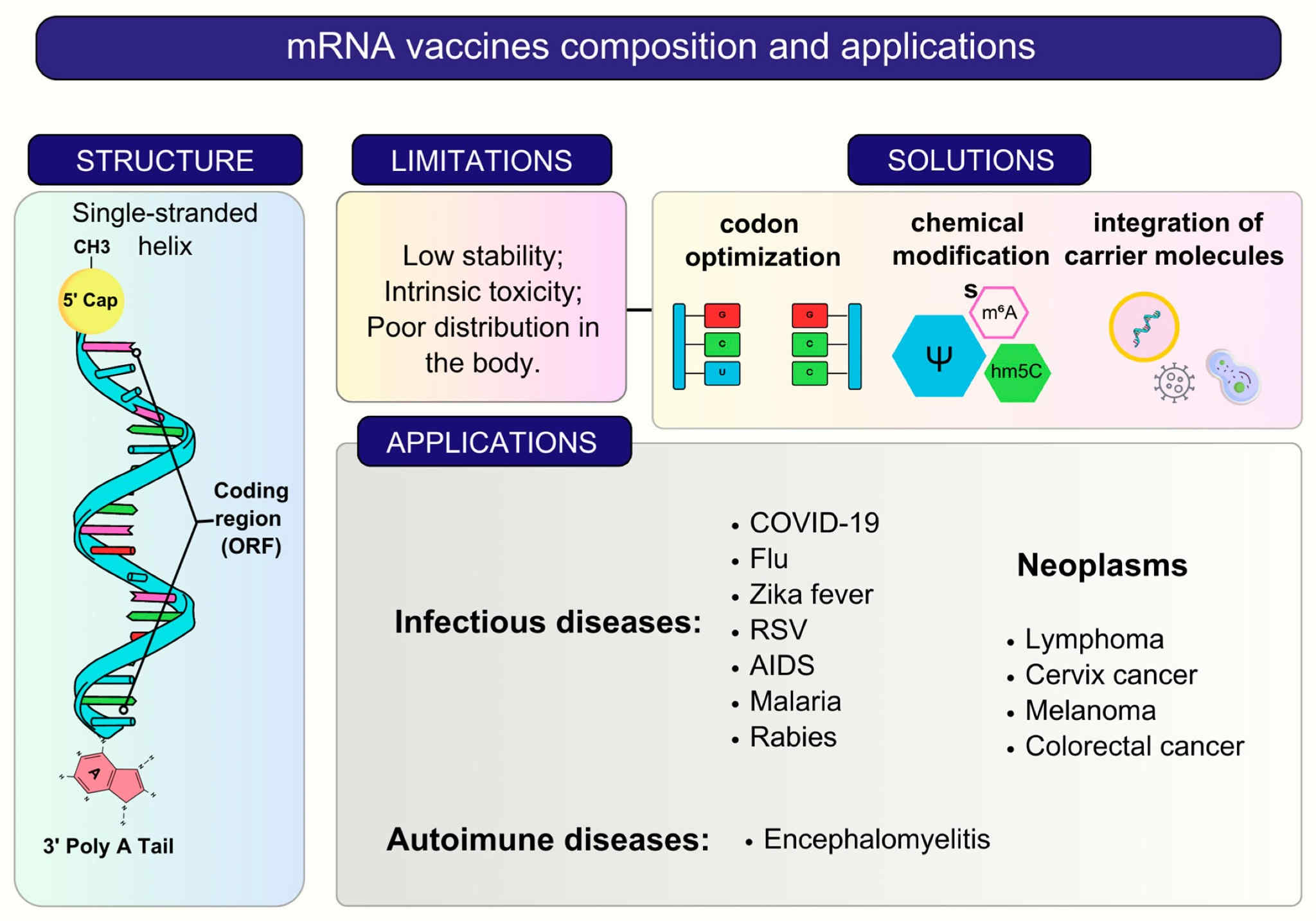 A general summary of composition, features, and applications of mRNA vaccines.2,5
A general summary of composition, features, and applications of mRNA vaccines.2,5
Dispensed in 96-well plate format as 10 mM stock solutions that are stable for months at −20 °C, chemical capping reagents allow robotic platforms to test hundreds of UTR variants or codon-optimized inserts without re-optimizing enzyme cocktails for each construct. Because there are no enzymatic steps, cap identity can be switched (Cap 0 → Cap 1 → Cap 2) by changing the reagent vial, facilitating orthogonal design-of-experiments that deconvolve the individual effects of cap methylation, modified nucleotides, and UTR length on translation efficiency . Microfluidic droplet generators take advantage of the one-pot nature of chemical capping to generate nanoliter-scale IVT reactions that use micrograms of reagent yet generate enough capped RNA for single-cell transfection studies. This flexibility speeds up early-stage lead selection and offers the statistical power to define design spaces that flow directly into clinical-scale DoE to ensure that the cap reagent chosen at the bench is the same one used in the final GMP batch.
Table 2 Application-specific selection of chemical capping reagents
| Application tier | Primary reagent class | Key functional attribute | Downstream advantage |
| High-throughput screening | ARCA dinucleotide | Orientation control | Rapid UTR/codon screens |
| Vaccine campaigns | Trinucleotide CleanCap | Co-transcriptional Cap-1 | Single-pot, metric-tonne scale |
| Personalized oncology | Custom trinucleotide | AG/CG/UG start codon | Parallel patient-specific synthesis |
| Rare-disease therapy | Extended-phosphate ARCA | Moderate DXO resistance | Balanced cost vs. half-life |
| Process development | Modular reagent set | Swap-on-demand | Direct scale-up path |
The choice of chemical cap reagent involves multiple considerations, such as molecular performance (efficiency, orientation purity), supply chain (vendor audits, second sourcing), and regulatory compliance (compendial status, DMF). As the cap directly impacts translational efficiency, immunogenicity, and stability, any variation in cap reagent quality can lead to inconsistent clinical potency. Thus, the cap should be considered as a critical raw material rather than a commodity, and its synthetic route, impurity profile, and long-term supply continuity should be carefully evaluated with the same rigor as the lipid nanoparticle.
Efficiency is dependent on the cap-to-GTP ratio, and on the analog's recognition by T7 RNA polymerase. The use of reagents that micelle or precipitate at high Mg2+ concentrations may lead to batch-to-batch swings from 85 % to <70 % forward incorporation, resulting in a twofold difference in protein expression. Reproducibility is therefore linked to crystalline morphology and residual solvent content: amorphous powders with >1 % water uptake may agglomerate during storage, with the consequence of non-homogeneous dissolution and unpredictable capping yields. Vendors must supply certificates of diastereomeric purity, endotoxin and trace-metal content, and must establish lot-to-lot CVs <5 % for capping rate, as determined by RP-HPLC or LC-MS. In-line Raman or UV probes can be used during IVT to monitor drift in real time, and adjust cap-to-GTP ratio before an entire batch is affected.
Chemical capping reagents are considered active pharmaceutical ingredients and thus regulated as critical raw materials according to ICH Q11. Therefore, each lot must be provided with a Certificate of Analysis (CoA), which should contain information on synthetic route, residual solvent profile, and mutagenic impurity assessment. The vendor should have ISO 9001 (or equivalent) quality certification for the production of clinical grade material, and should be able to provide a Drug Master File (DMF) or equivalent regulatory package. Of particular importance for ARCA-type analogs is diastereomeric purity, as the wrong isomer can lead to a chain terminator product. Specification limits of <1 % are commonly required. Endotoxin must be <0.25 EU/mL and heavy-metal residues (Pd, Pt from coupling chemistry) must meet specification according to ICH Q3D limits. Accelerated stability data (40 °C/75 % RH for six months) must be available to back up any shelf-life claims. Finally, the reagent must be compatible with downstream purification, i.e. it should not form adducts with modified nucleotides and it should not interact with anion-exchange chromatography, as any interaction would require validation of removal.
The short supply risk for capping reagents is recognized and is considered a single-source risk due to multi-step phosphorylation chemistry that is difficult to transfer. Two or more qualified vendors, with orthogonal synthetic routes (e.g. one vendor with a phosphoramidite coupling and one with P-imidazolide activation) should be qualified in the event that one synthetic route cannot clear regulatory review. Business continuity of the vendors, including at least six to twelve months of finished goods inventory and a documented second source qualification package should be submitted by the vendor. Diversification of geographic location of synthesis is important, as reagents that are made in a country that has unpredictable export laws or limited number of GMP facilities have the potential to affect global launch programs. Long-term contracts with lock-in price and force majeure provisions that assure top-priority product allocation during pandemic level surges should be considered. The vendor's quality system should include change-control notifications if there is a change in starting material, solvent, or purification resin, as small changes in these areas may impact diastereomeric purity and potentially invalidate previous toxicology packages.
Chemical capping increases raw-material cost by 15–30 % over uncapped IVT, but the performance gain can reduce the effective cost-per-dose by allowing lower RNA and lipid input to reach a given pharmacodynamic endpoint. The TCO calculation needs to consider not only the price of reagents but also the avoided costs of enzymatic capping (enzyme purchase, additional QC, buffer exchange) and reduced risk of batch failure due to incomplete methylation. If pricing is based on an agreed targeted use level, then volume-based tiered pricing and multi-year supply agreements for high-volume products can fix the cost below the known volatility in enzyme markets. For early-stage or personalized products, the premium paid for trinucleotide Cap-1 analogs can be more than offset by the accelerated IND timelines and complete removal of enzyme-related CMC questions. In the end, the selected reagent must provide the lowest functional cost per patient dose and pass all purity, regulatory, and supply-security gates, and the value equation makes chemical capping an attractive performance and economic enabler.
We offer a robust portfolio of chemical capping reagents designed to support efficient and reproducible et al. transcription (IVT) mRNA production. Our solutions enable co-transcriptional capping with high incorporation efficiency, simplified workflows, and strong process control, making them suitable for both research and large-scale mRNA manufacturing. Each reagent system is developed with a focus on performance, consistency, and compatibility with commonly used IVT enzymes and reaction conditions.
Our high-efficiency chemical capping reagents are optimized to maximize capping rates during IVT, resulting in a high proportion of properly capped mRNA. By supporting efficient co-transcriptional capping, these reagents help improve mRNA stability and translation efficiency while reducing the need for additional post-transcriptional processing steps. They integrate seamlessly into standard IVT workflows and are compatible with widely used RNA polymerases.
We provide GMP-grade chemical capping reagents manufactured under controlled conditions to meet the quality and documentation expectations of regulated mRNA production. These reagents are developed with an emphasis on consistent composition, low impurity profiles, and reliable performance, supporting robust process development and long-term manufacturing workflows.
In addition to our standard offerings, we support custom chemical capping formulations to address specific process requirements, such as unique reaction conditions or integration into established IVT platforms. Our technical team works closely with customers to optimize reagent composition and usage parameters, helping ensure efficient capping performance and smooth process implementation.
Our chemical capping reagents have demonstrated reliable performance in scaled IVT mRNA production, maintaining high capping efficiency and reproducibility as reaction volumes increase. This consistency supports smooth scale-up, reduces batch-to-batch variability, and helps maintain stable mRNA quality throughout manufacturing workflows.
Chemical capping offers a streamlined and scalable approach to improving capping efficiency, workflow simplicity, and overall mRNA quality, but optimal results depend on selecting the right reagents and conditions. Whether you are refining an existing IVT process or scaling up mRNA production, our team can help you identify and implement the most effective chemical capping solution—contact us today to discuss your workflow and request technical guidance or a quotation.
References
Chemical capping reagents enable co-transcriptional introduction of the 5' cap during IVT mRNA synthesis.
It combines transcription and capping into a single step, reducing process complexity and handling time.
Chemical capping can achieve high capping efficiency when reaction conditions are properly optimized.
Yes, chemical capping is widely used in scaled IVT workflows due to its reproducibility and simplicity.
Cap analog type, cap-to-NTP ratio, enzyme selection, and reaction conditions all influence performance.

