Orientation is not a nuance—it is the single most important variable distinguishing a translationally active transcript from a dead RNA. Standard dinucleotide cap analogs (M7 GpppG) leave both guanosines with free 3'-OH groups, permitting T7 or SP6 polymerase to start extension from either end. Approximately one-third to one-half of the resulting molecules therefore have the cap backwards (Gppp-m7G), which eIF4E cannot grip and that is rapidly targeted by decapping enzymes. Anti-reverse cap analogs (ARCAs) remove this uncertainty by methylating (or eliminating) the 3'-OH of the m7G ribose, thereby forcing elongation to begin exclusively from the unblocked guanosine. The result is a population in which every transcript presents the cap in the native, forward orientation, translating more efficiently and persisting longer in cells. Thus, orientation is not just important—it is the primary reason ARCA-capped mRNAs outperform their standard-capped counterparts across virtually every downstream metric.
Orientation of the cap in in vitro transcribed mRNA is another important quality attribute as it impacts translational competence and activity. The 5' cap stereochemistry is essential for recognition by the translation machinery, for instance by the selective binding of eukaryotic initiation factor 4E (eIF4E) to the properly oriented 7-methylguanosine. The orientation of the cap depends on which hydroxyl group of the cap dinucleotide is used for the RNA polymerase extension. As both the 2' and 3' hydroxyl groups of the methylated guanosine are available for phosphodiester linkage with the rest of the transcript in standard cap analogs, two populations with opposite orientations are formed. Only when the 7-methylguanosine is at the terminal position is the cap properly oriented to support translation. Transcripts with the improper orientation of the cap are, in terms of translation efficiency and stability, similar to uncapped RNA. The presence of two different orientations of capped RNA therefore severely reduces the amount of functional mRNA and, consequently, the protein expression yield. Moreover, it may induce cell quality control pathways that target the aberrant cap structures.
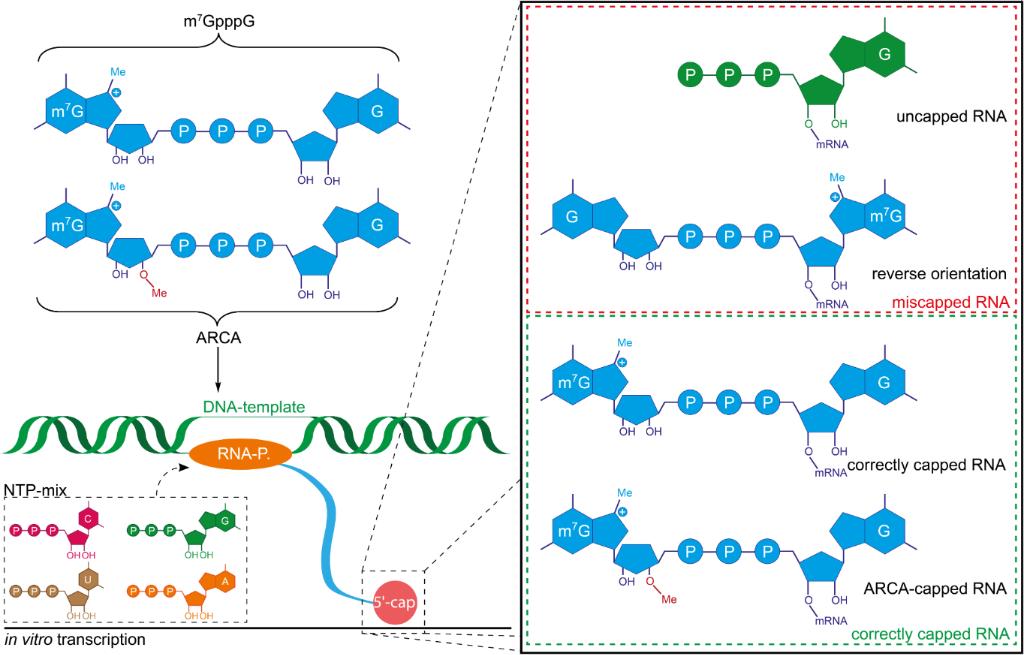
Reverse cap incorporation is a serious and common problem with in vitro mRNA capping. It is the insertion of the cap dinucleotide in the reverse orientation, which makes the capped transcript translationally inactive. This can occur during co-transcriptional capping if the RNA polymerase binds the cap analog in the wrong orientation, starting transcription with the wrong 3' or 5' hydroxyl. In this case, the 7-methylguanosine unit ends up in the middle of the molecule, instead of the 5' end, with a normal phosphodiester backbone linking the first transcribed nucleotide to the 3' hydroxyl of the cap analog. The capped transcript thus has the proper chemical identity but the wrong stereochemistry to be recognized by eukaryotic initiation factor 4E. In a reverse-transcript, the phosphodiester bond is between the 5' carbon of the first transcribed nucleotide and the 3' carbon of the 7-methylguanosine, with an inverted 5'-3' linkage. The cap-binding protein will not bind to this structure, as it is the exact stereochemical opposite of the correct structure. The result is the same as an uncapped transcript: it will be subject to rapid degradation by 5'-to-3' exonucleases, and virtually no protein expression, even though the transcript is chemically capped. The fraction of reverse incorporation depends on the cap analog and conditions used, but can be a large fraction of the total capped transcripts using conventional cap analogs. The presence of both orientations also complicates downstream purification of the capped transcripts, as they can not be physically separated from the correctly oriented product. Anti-reverse cap analogs have been developed to address this issue, and result in essentially all caps being in the functional orientation.
Standard cap analogs, although more easily made and implemented, have a variety of shortcomings when used to produce synthetic mRNA. Standard cap analogs suffer from orientation heterogeneity and this results in a synthetic dead-end and reduced biological activity compared to more advanced analogs. The most commonly used standard cap analog is M7 GpppG which is incorporated into the 5' end of RNA in vitro in a mixture of 1:1 ratios during transcription elongation, and is dependent on whether the RNA polymerase extends from the 2' or 3' hydroxyl group of the first nucleotide that is methylated on the guanine. This means that for every two caps that are incorporated, one will have the orientation shown in Scheme 1, and the other will be the mirror image. As a result, one half of the capped RNA molecules are synthesized as though uncapped as the wrong orientation of the cap inhibits cap function. Standard cap analogs in general produce only Cap 0 analogs which have poor immune evasion, as the 2'-O-methylation found in natural mRNA is absent. As a result, many synthesis workflows using standard analogs require additional enzymatic treatment to convert Cap 0 analogs to Cap 1 analogs, driving up cost and process complexity. In order to ensure that a higher proportion of caps are added, the ratio of cap analog to GTP is usually made high, driving up cost of raw materials while still not producing exclusively capped RNA. Furthermore, standard cap analogs are less stable in cell culture media, making them less suited to translation as they are more sensitive to decapping enzymes, Dcp1/Dcp2, which will prematurely degrade capped RNA.
Table 1 Orientation-Dependent Performance Metrics.
| Cap Analog Type | Orientation Control | Cap Structure Generated | Translation Efficiency | Primary Limitation |
| Standard M7 GpppG | Mixed orientations | Cap 0 | Moderate | 50% reverse orientation |
| ARCA | Correct orientation only | Cap 0 | High | Still requires Cap 1 conversion |
| Advanced ARCA | Correct orientation | Cap 1 | Superior | Higher synthetic complexity |
Anti-reverse cap analogs (ARCA) are small molecule dinucleotides that address the orientation heterogeneity in the cap structure. The 3'-OH on the N7-methylguanosine ribose is blocked to force elongation to begin only from the second, free guanosine residue. The single steric change transforms a random 50:50 mix of forward- and reverse-capped transcripts to a nearly homogenous population of native-like mRNA in one simple step and without any additional unit operations. Mechanistically, ARCA is a suicide primer: extension from the methylated end stalls the polymerase upon reaching a dead-end hydroxyl and leads to abortion. Initiation must therefore occur in the correct orientation, producing m7G(5')ppp(5')G-RNA that can bind eIF4E and resist decapping. Subsequent generation ARCAs modify the phosphate bridge with phosphorothioate or methylene linkages to further cap-armour transcripts against hydrolysis while still maintaining the orientation lock for a plug-and-play reagent that boosts both translational output and in-cell half-life in a single IVT step.
 Schematic representation of co-transcriptional capping with different cap analogues.1,5
Schematic representation of co-transcriptional capping with different cap analogues.1,5
Structural changes in ARCA have been limited to changes in the ribose of the 7-methylguanosine residue that ablate one of the two ways the molecule can be incorporated to give the single active cap analog (reverse incorporation). The canonical ARCA contains a methylation of the 3'-hydroxyl group of the 7-methylguanosine. A methyl group replaces the -OH leaving a methoxy group which is unable to form the new phosphodiester bond in the initiation reaction. This change leaves the overall structure of the cap intact with only a single atomic difference between the two faces of the analog. Removal of the -OH leaving only a hydrogen is also a sterically occlusive change. The addition of a 2'-O-methyl group has also been shown to block reverse incorporation and might have a higher affinity for eIF4E. Phosphorothioate modifications in the triphosphate bridge have been shown to make the cap stable to decapping enzymes. Phosphate bridges as long as hexaphosphate have been synthesized and are thought to block the reverse orientation while leaving the recognition motifs of the cap intact and potentially increasing the binding to eIF4E. These larger steric changes in the cap must be carefully tuned so that recognition by the translation machinery does not suffer. Crystal structures have shown the 2' and 3' positions to be solvent accessible and not to play a large role in protein binding of the methylated guanosine.
The prevention of reverse incorporation is the main functional rationale for ARCA. As can be seen in Figure 3, typical cap analogs contain two hydroxyl groups (2' and 3') available for initiation on the methylated guanosine. Enzymes such as RNA polymerase may therefore initiate from either side, leading to cRNA that is capped on both the 5' and 3' termini. ARCA by contrast is designed such that only one hydroxyl group is available as a target for incorporation, and therefore once the guanosine is added, the enzyme is forced to use the remaining hydroxyl group for initiation. As this modification is chemically based, it is resistant to mutations in polymerase that might otherwise lead to transcription in both directions. The incorporation is prevented because there is no free hydroxyl group with which to form the initial phosphodiester bond in that direction; by removing one of the two possible handles the polymerase must start and finish from the same side. The use of ARCA also ensures the 7-methylguanosine is always found in the 5' terminal position on mRNA where it can be bound by cap-binding proteins and translation initiation factors. This functional specificity has been observed experimentally and suggests that the efficiency of ARCA-capped transcripts is only slightly less than enzyme capped mRNA. By preventing the creation of both functional and non-functional transcripts, reverse incorporation also reduces the need for separation of properly oriented cRNA. New mechanistic data has also shown that orientation control is similar across a variety of promoter sequences and reaction conditions, and is therefore robust to changes in the production environment.
Table 2 Comparison of different Caps
| Cap Analog Type | Orientation Control | Mechanism | Functional Outcome |
| Standard M7 GpppG | Mixed orientations | Two reactive hydroxyl groups | ~50% non-functional transcripts |
| ARCA (3'-O-Me) | Correct orientation only | Blocked 3'-hydroxyl | Nearly 100% functional orientation |
| Alternative ARCA | Correct orientation | 2'-modification or deoxygenation | Equivalent orientation control |
Direct comparisons with standard cap analogs show that ARCA offers significant performance benefits, making it the preferred choice for applications where the highest level of mRNA activity is required. The key difference between standard M7 GpppG and ARCA is the control over orientation. Standard M7 GpppG results in a mixture of molecules, with about half of the transcripts being in the non-functional reverse orientation. ARCA, on the other hand, enforces correct orientation during incorporation, resulting in virtually all capped mRNA being in the correct orientation to interact with the translational machinery. This results in improved translation efficiency, higher protein expression levels, and increased biological activity in various experimental systems. The performance advantage of ARCA is especially pronounced in cases where the highest possible expression is needed, particularly in challenging cellular environments. Recent studies directly comparing ARCA and standard cap analogs head-to-head have demonstrated the consistently superior performance of ARCA, with the degree of improvement correlating with the reduction of transcripts in the reverse orientation.
ARCA also has been reported to be more efficient than standard cap analogs. Standard cap analogs, such as M7 GpppG, produce caps in both the + and - orientation, with only the + orientation being able to recruit eukaryotic translation initiation factor 4E and initiate translation. This gives an effective 50% loss of translationally competent cap analog. ARCA, due to its fixed orientation, caps almost exclusively in the correct orientation and as a result should have a higher rate of ribosome loading. This has been demonstrated with ARCA capped transcripts showing higher rates of protein synthesis in a wide range of cell lines and experimental conditions. The translation efficiency has been reported to be higher in primary cells and in vivo. A recent publication showed that ARCA capped mRNA translation efficiencies to be comparable to those of enzymatic capped mRNAs, while still retaining the benefits of chemical cap installation. The higher translation efficiency is also seen as less time between transfection and the first detectable expression of the protein of interest, which can be of great importance in industrial applications where a fast production of protein is required. Newer derivatives of ARCA have been synthesized with additional modifications that have led to an even higher translation efficiency. One of these analogs showed a 6-fold increase in translation efficiency.
As expected, the improved translational efficiency is also reflected in the level of protein expression. ARCA-capped mRNAs produce a larger amount of protein over a wide range of experimental systems and time points. This is true for the direct comparison of ARCA- to the regular analog capped mRNA where the increase in protein expression is on the order of several-fold and depends on the fraction of properly oriented caps present. The increased expression remains constant over long time courses, suggesting that the improved translation efficiency works in concert with improved mRNA stability to enhance the total amount of protein produced. The higher expression is particularly marked in primary cells with intact innate immune systems, where ARCA's lack of immunogenicity is an added advantage to its translation enhancement. The use of ARCA derivatives with further improved stabilization elements has resulted in further improvements in expression, with some cap analogs tested resulting in several fold increases in total protein expression relative to the standard cap analog. The improved expression is not mRNA or protein specific and has been observed across a variety of different mRNA sequences and protein products, suggesting that this improvement is the result of the change in the cap structure rather than specific sequence preferences. This has obvious benefit to assay sensitivity, minimum mRNA dose and more robust experiments in both research and therapeutic applications. The translation efficiency advantage of ARCA is observed across a number of different proteins from the commonly used reporter proteins to therapeutic proteins.
Experimental Overview: A wealth of experimental evidence has consistently shown the superior performance of ARCA relative to other capping analogs and approaches. These findings have been observed across various experimental systems and methodologies, and have been validated in multiple independent studies. ARCA-capped transcripts demonstrate increased translation efficiency in cell-free and cell-based assays, and this improvement is consistently observed across different mRNA sequences and experimental conditions. The increased protein expression from ARCA-capped mRNA is robust and quantifiable, with the degree of improvement often correlating with the removal of antisense or negative sense oriented transcripts that negatively impact the performance of traditional analogs. ARCA-capped mRNA has been found to be more resistant to decapping enzymes and exhibit increased intracellular stability, leading to prolonged protein expression over time. Experimental evidence supporting the advantages of ARCA also extends to in vivo models, with improved performance observed in tissue-specific expression studies and therapeutic applications. Advanced analytical methods, such as single molecule imaging and quantitative PCR, have been employed to gain mechanistic insights into the enhanced translation initiation and prolonged mRNA half-life conferred by ARCA. Recent comprehensive studies have validated the performance benefits of ARCA across different cell types, including primary cells and professional antigen-presenting cells, confirming the broad applicability of these improvements. Overall, the collective experimental evidence firmly establishes ARCA as the gold standard for chemical capping in applications where the highest level of performance is desired, while also informing the development of next-generation analogs that build upon the foundational improvements seen with ARCA.
Pick ARCA when the expense of a cap analogue is outstripped by the value of predictable, high-level protein production - a range of applications from recalcitrant low-expressing genes to multi-dose therapeutics. The single-atom modification (3'-OH blockade on m7G) transforms a racemic cap mixture into an almost homogenous population of forwards-facing molecules, providing a disproportionately high increase in translatable molecules without adding steps to the workflow. The choice matrix thus hinges on risk of expression failure: if batch-to-batch variation, immunogenicity, or dose sparing would threaten the project, ARCA is the default and not the upgrade.
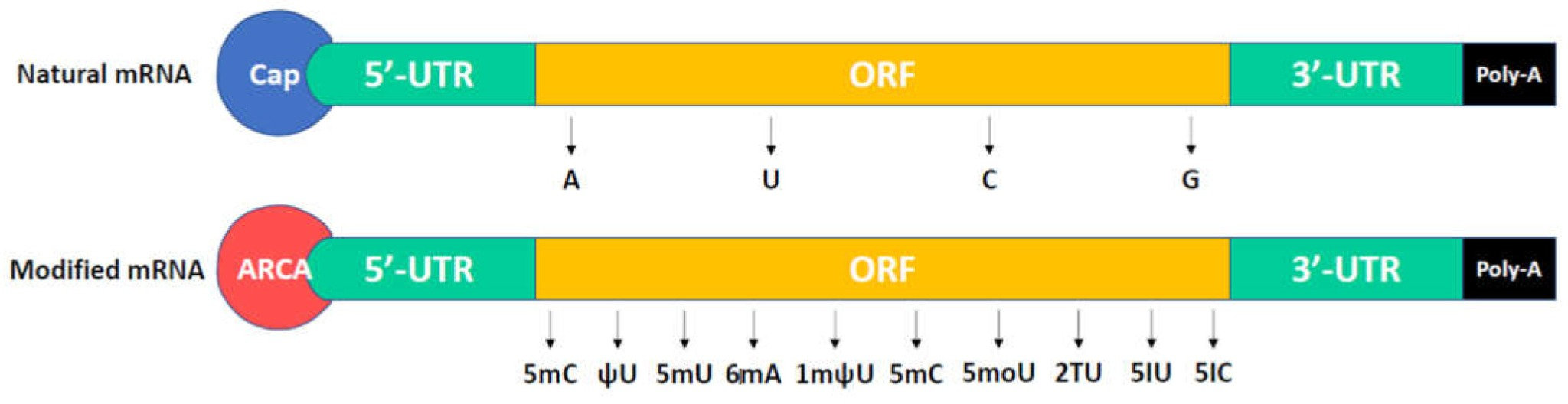 The structure of a mature eukaryotic mRNA and a modified mRNA.2,5
The structure of a mature eukaryotic mRNA and a modified mRNA.2,5
Difficult-to-express proteins are the most obvious application for ARCA since the dramatic improvement in translation will be more than enough to overcome any intrinsic defects that may be limiting production of the protein of interest from conventional mRNA preparations. This could include membrane proteins, large multi-domain enzymes, or proteins whose folding is sensitive to the rate of expression. The fact that ARCA maximizes the number of functional mRNAs also means that proteins with low translational efficiencies can also be expressed. Of course, orientation control is most important for these difficult to express proteins, since doubling the effective yield of the protein will be critical for proteins that are close to the limit of expression detectability. One recent example of this use case was in cellular reprogramming where the use of ARCA was particularly important to control the expression levels of key transcription factors to successfully convert to a target lineage. The improved kinetics of expression is also useful for proteins where fast accumulation is required to reach the threshold for functionality, such as many enzymes used for metabolic engineering or signaling proteins that need to accumulate to a critical concentration to drive activation of a signaling cascade. ARCA also allows tuning the expression level of proteins that are toxic at high concentrations, through the ability to lower the total mRNA dose while still achieving high protein expression levels for functional studies.
The purpose of use will often dictate the use of ARCA or not. In a research setting, one must carefully consider whether the improved performance of ARCA justifies the increased cost for a particular application. The use of ARCA is likely to be justified for experiments where high precision is necessary and expression levels will be critical for interpretation, such as mechanistic studies, or for certain applications involving precious biological material where there is a need to get the highest possible yield of function. The improved translation efficiency of ARCA can also be useful for high throughput screening (HTS) to get a better signal-to-noise ratio, possibly allowing one to detect hits which would not be picked up by the standard analog. On the other hand, for therapeutic applications, the use of ARCA is almost a foregone conclusion, given the need for high and predictable quality of the product and maximal performance. Translation into the clinic of mRNA therapeutics would likely require predictable and robust expression profiles which are not achievable by the standard analog. Calculation of therapeutic dose based on protein expression will have to take into account the fact that a significant fraction of the transcript produced by standard analog will be non-functional, therefore the level of control over orientation by ARCA will be needed for the therapeutic index to be met. Moreover, the lower immunogenicity of the more homogeneous, correctly capped transcripts will be necessary to meet safety criteria for human use. When spread out over the number of doses in a therapeutic regimen, the cost differential may become insignificant, and the performance advantages translate directly to the clinic in the form of better efficacy and lower doses.
Anti-reverse cap analogs (ARCA) are specifically designed to control cap orientation during in vitro transcription (IVT), ensuring that the 5' cap is incorporated in a translation-competent configuration. Our ARCA products are developed to address the limitations of standard cap analogs by reducing reverse incorporation, thereby increasing the proportion of correctly capped mRNA and improving overall translation performance. Each product is manufactured with an emphasis on quality, reproducibility, and compatibility with established IVT workflows.
Our high-purity ARCA products are optimized for efficient co-transcriptional incorporation during IVT mRNA synthesis. By preventing reverse cap orientation, these ARCA reagents help maximize effective ribosome recognition and support higher protein expression levels. Consistent chemical purity and low impurity profiles ensure reliable performance across commonly used IVT systems, making them well suited for mRNA optimization and process development.
We also offer GMP-grade ARCA supply options manufactured under controlled quality systems to support regulated mRNA production workflows. These materials feature stringent quality control, batch traceability, and comprehensive documentation, helping maintain consistent cap orientation and reproducible translation efficiency across extended manufacturing campaigns.
Cap orientation plays a critical role in determining how effectively mRNA is translated, and selecting the right cap analog can significantly influence protein expression outcomes. If you are seeking to improve translation efficiency and reduce variability associated with reverse cap incorporation, our ARCA solutions can help—contact us today to discuss your IVT mRNA workflow and request technical support or a quotation.
References
Cap orientation refers to whether the cap is incorporated in a translation-competent configuration.
Standard cap analogs can be incorporated in either orientation during IVT.
ARCA is chemically modified to prevent reverse incorporation.
Yes, only correctly oriented caps support efficient translation.
ARCA is most beneficial when high translation efficiency is required.

